|
|
Developmental Biology - CRISPR
Finding Leukemia's Weakness Using CRISPR
Using CRISPR to identify and genetically delete Stau2 protein, led to profound reduction in leukemia growth...
A team of researchers at University of California San Diego School of Medicine and Moores Cancer Center used CRISPR technology to identify key regulators of aggressive chronic myeloid leukemia, a type of cancer that remains difficult to treat and is marked by frequent relapse.
"We used CRISPR technology to carry out a genome-wide screen in leukemia cells to block thousands of genes at once. This is an extremely powerful tool that allowed us to identify a multitude of genes that fuel leukemia growth and find new vulnerabilities that can be targeted in this disease.
The study also shows, for the first time, that whole genome CRISPR-based screens can be carried out to be much more physiologically relevant: using primary cancer cells — in the setting of their native microenvironment."
Tannishtha Reya PhD, Professor, Departments of Pharmacology and Medicine, University of California, San Diego School of Medicine, Moores Cancer Center and senior author.
Reporting in the April 20, 2020 online edition of the journal Nature Cancer, Reya and colleagues identified RNA-binding proteins - which normally control how, when and if cells make certain proteins - as a key class of proteins that sustain and protect drug-resistant leukemia stem cells.
The authors focused on Staufen2 (Stau2), a relatively understudied member of the RNA-binding protein family that was previously only known to control brain and nervous system development.
This work was carried out in collaboration with UC San Diego investigators Gene Yeo, PhD, and Anjana Rao, PhD, and was conducted by lead author Jeevisha Bajaj, PhD, while she was a postdoctoral fellow at UC San Diego in the Reya lab. Bajaj subsequently joined the University of Rochester Medical Center as assistant professor of biomedical genetics and a researcher at the Wilmot Cancer Institute.
The team developed a mouse model in which Stau2 was genetically deleted and found that loss of this protein led to a profound reduction in leukemia growth and propagation, and markedly improved overall survival in mouse models. Stau2 was also required for continued growth of primary tissue samples of patients with leukemia, indicating a conserved dependence in the human disease.
"We are particularly excited about this work because, to our knowledge, this is the first demonstration that Staufen2 is a key dependency in any cancer."
Tannishtha Reya PhD.
To understand how Stau2 controls cancer, researchers undertook a genome-scale computational analysis of its targets through RNA-Seq and eCLIP-Seq. This led to the discovery that this protein controls key oncogenes, such as Ras, and epigenetic regulators, such as the LSD/KDM family of proteins, which are critical drug targets being tested against leukemia and other cancers.
According to the National Cancer Institute, approximately 1.5 percent of men and women will be diagnosed with leukemia at some point during their lifetimes. While chronic myeloid leukemia (CML) can be controlled with targeted therapies, this disease can be lethal if it advances or is diagnosed in an acute "blast" phase. The findings also have implications for acute myeloid leukemia (AML) and other blood cancers.
"This work will be particularly important for the discovery of new treatments. Our genome-wide screen identified cellular signals critical for the growth of cancer, and in the future, this study will be useful to study the microenvironment, the area around the tumor that includes tissue, blood vessels and important molecular signals related to how the cancer behaves."
Jeevisha Bajaj PhD, Department of Pharmacology, University of California San Diego, School of Medicine, La Jolla, CA, USA.
Abstract
Aggressive myeloid leukemias such as blast crisis chronic myeloid leukemia and acute myeloid leukemia remain highly lethal. Here we report a genome-wide in vivo CRISPR screen to identify new dependencies in this disease. Among these, RNA-binding proteins (RBPs) in general, and the double-stranded RBP Staufen2 (Stau2) in particular, emerged as critical regulators of myeloid leukemia. In a newly developed knockout mouse, loss of Stau2 led to a profound decrease in leukemia growth and improved survival in mouse models of the disease. Further, Stau2 was required for growth of primary human blast crisis chronic myeloid leukemia and acute myeloid leukemia. Finally, integrated analysis of CRISPR, eCLIP and RNA-sequencing identified Stau2 as a regulator of chromatin-binding factors, driving global alterations in histone methylation. Collectively, these data show that in vivo CRISPR screening is an effective tool for defining new regulators of myeloid leukemia progression and identify the double-stranded RBP Stau2 as a critical dependency of myeloid malignancies.
Authors
Jeevisha Bajaj, Michael Hamilton, Yutaka Shima, Kendall Chambers, Kyle Spinler, Eric L. Van Nostrand, Brian A. Yee, Steven M. Blue, Michael Chen, David Rizzeri, Charles Chuah, Vivian G. Oehler, H. Elizabeth Broome, Roman Sasik, James Scott-Browne, Anjana Rao, Gene W. Yeo and Tannishtha Reya.
Acknowledgements
The authors are grateful to S. Levi for technical support and M. Kritzik for help with manuscript preparation. We thank P. Adams and P.M. Vertino for scientific advice, W. Pear (University of Pennsylvania) and A.M. Pendergast (Duke University) for the BCR-ABL construct and D.G. Gilliland for the NUP98-HOXA9 construct. J.B. is a recipient of a Scholar Award from the American Society of Hematology and a postdoctoral fellowship from the National Cancer Center. M.H. was supported by the National Institutes of Health (NIH) Training Grant T32HL086344 and K.S. received support from NIH Training Grants T32HL086344 and T32CA009523. E.L.V.N. was a Merck Fellow of the Damon Runyon Cancer Research Foundation (DRG-2172-13) and is supported by the National Human Genome Research Institute (HG009530). J.S.-B. was the Fraternal Order of Eagles Fellow of the Damon Runyon Cancer Research Foundation. This work was supported by NIH grants R35 CA210043 awarded to A.R; U54HG007005 and U41HG009889 awarded to G.W.Y.; and DK099335, DP1 CA174422 and R35 CA197699 awarded to T.R.
Return to top of page.
| |
|
Apr 24 2020 Fetal Timeline Maternal Timeline News
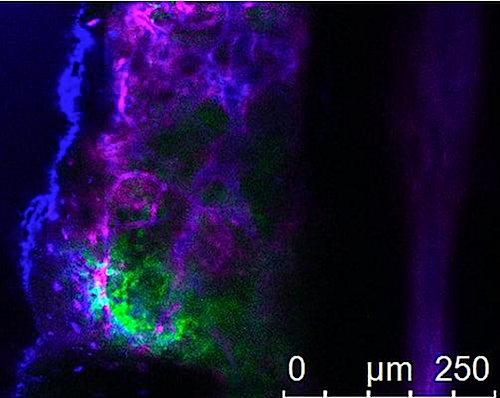 UC San Diego researchers used CRISPR technology to carry out a genome-wide screen in leukemia cells to block thousands of genes at once. The tool was used to identify genes that fuel leukemia growth, like those leukemia cells (GREEN) pictured growing within bone marrow. CREDIT UC San Diego Health Sciences.
|



