|
|
Developmental Biology - CRISPR Correction
Diabetes Reversed In Mice!
CRISPR corrects genetic defect in mice so that cells can normalize blood sugar...
Using induced pluripotent stem cells collected from a patient with Wolfram syndrome - a rare, genetic form of insulin dependent diabetes - researchers transformed human stem cells into mouse insulin-producing cells.
They then used the gene-editing tool CRISPR-Cas9 to correct the genetic defect causing Wolfram thus curing diabetes in Wolfram syndrome mice.
These findings from researchers at Washington University School of Medicine in St. Louis, Missouri, suggest the CRISPR-Cas9 technique may hold promise as a treatment for diabetes. Particularly diabetes forms caused by a single gene mutation, and it also may be useful one day in some patients with the more common forms of diabetes, such as type 1 and type 2.
The study published online April 22 in the journal Science Translational Medicine.
Patients with Wolfram syndrome develop diabetes during childhood or adolescence and quickly require insulin-replacement therapy, requiring insulin injections multiple times each day. Most go on to develop problems with vision and balance, as well as other issues, and in many patients, the syndrome contributes to an early death.
"This is the first time CRISPR has been used to fix a patient's diabetes-causing genetic defect and successfully reverse diabetes. For this study, we used cells from a patient with Wolfram syndrome because, conceptually, we knew it would be easier to correct a defect caused by a single gene. But we see this as a stepping stone toward applying gene therapy to a broader population of patients with diabetes."
Jeffrey R. Millman PhD, Assistant Professor, Departments of Medicine and Biomedical Engineering, Washington University, St Louis, Missouri, USA; and Co-Senior Investigator.
Wolfram syndrome is caused by mutations to a single gene, providing the researchers an opportunity to determine whether combining stem cell technology with CRISPR to correct the genetic error also might correct the diabetes caused by the mutation.
A few years ago, Millman and his colleagues discovered how to convert human stem cells into pancreatic beta cells. When such cells encounter blood sugar, they secrete insulin. Recently, those same researchers developed a new technique to more efficiently convert human stem cells into beta cells that are considerably better at controlling blood sugar.
In this study, they took the additional steps of deriving these cells from patients and using the CRISPR-Cas9 gene-editing tool on those cells to correct a mutation to the gene that causes Wolfram syndrome (WFS1). Then, the researchers compared the gene-edited cells to insulin-secreting beta cells from the same batch of stem cells that had not undergone editing with CRISPR.
In the test tube and in mice with a severe form of diabetes, the newly grown beta cells that were edited with CRISPR more efficiently secreted insulin in response to glucose. Diabetes disappeared quickly in mice with the CRISPR-edited cells implanted beneath the skin, and the animals' blood sugar levels remained in normal range for the entire six months they were monitored. Animals receiving unedited beta cells remained diabetic. Their newly implanted beta cells could produce insulin, just not enough to reverse their diabetes.
"We basically were able to use these cells to cure the problem, making normal beta cells by correcting this mutation. It's a proof of concept demonstrating that correcting gene defects that cause or contribute to diabetes - in this case, in the Wolfram syndrome gene - we can make beta cells that more effectively control blood sugar. It's also possible that by correcting the genetic defects in these cells, we may correct other problems Wolfram syndrome patients experience, such as visual impairment and neurodegeneration."
Fumihiko Urano MD PhD, Co-senior Investigator; Samuel E. Schechter Professor of Medicine, Professor of Pathology and Immunology, Washington University, St Louis, Missouri.
In the future, using CRISPR to correct certain mutations in beta cells may help patients whose diabetes is the result of multiple genetic and environmental factors. Type 1 diabetes is caused by an autoimmune process that destroys beta cells. While type 2 diabetes, is closely linked to obesity and a systemic process called insulin resistance.
Millman adds: "We're excited about the fact that we were able to combine these two technologies - growing beta cells from induced pluripotent stem cells and using CRISPR to correct genetic defects. In fact, we found that corrected beta cells were indistinguishable from beta cells made from the stem cells of healthy people without diabetes."
The process of making beta cells from stem cells should get easier, the researchers said. For example, the scientists have developed less intrusive methods, making induced pluripotent stem cells from blood - and they are working on developing stem cells from urine samples.
"In the future, we may be able to take a few milliliters of urine from a patient, make stem cells that we then can grow into beta cells, correct mutations in those cells with CRISPR, transplant them back into the patient, and cure their diabetes in our clinic. Genetic testing in patients with diabetes will guide us to identify genes that should be corrected, which will lead to a personalized regenerative gene therapy."
Jeffrey R. Millman PhD
Abstract
Differentiation of insulin-producing pancreatic ? cells from induced pluripotent stem cells (iPSCs) derived from patients with diabetes promises to provide autologous cells for diabetes cell replacement therapy. However, current approaches produce patient iPSC-derived ? (SC-?) cells with poor function in vitro and in vivo. Here, we used CRISPR-Cas9 to correct a diabetes-causing pathogenic variant in Wolfram syndrome 1 (WFS1) in iPSCs derived from a patient with Wolfram syndrome (WS). After differentiation to ? cells with our recent six-stage differentiation strategy, corrected WS SC-? cells performed robust dynamic insulin secretion in vitro in response to glucose and reversed preexisting streptozocin-induced diabetes after transplantation into mice. Single-cell transcriptomics showed that corrected SC-? cells displayed increased insulin and decreased expression of genes associated with endoplasmic reticulum stress. CRISPR-Cas9 correction of a diabetes-inducing gene variant thus allows for robust differentiation of autologous SC-? cells that can reverse severe diabetes in an animal model.
Authors
Maxwell KG, Augsornworawat P, Velazco-Cruz L, Kim MH, Asada R, Hogrebe NJ, Morikawa S, Urano F, Millman JR. Gene-edited human stem cell-derived ß cells from a patient with monogenic diabetes reverse pre-existing diabetes in mice. Science Translational Medicine, published online April 22, 2020.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of General Medical Sciences, the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Grant numbers R01 DK114233, DK112921, TR002065, TR002345, T32 DK108742, R25 GM103757, T32 DK007120, P30 DK020579, P30 CA91842, UL1 TR000448 and UL1 TR002345. Additional assistance was provided by the Washington University Genome Engineering and iPSC Center, the Washington University Diabetes Center, and the Washington University Institute of Clnical and Translational Science, with additional funding from the JDRF, the Washington University Center of Regenerative Medicine, startup funds from the Washington University School of Medicine Department of Medicine, the Unravel Wolfram Syndrome Fund, Silberman Fund, Stowe Fund, Ellie White Foundation for Rare Genetic Disorders, Eye Hope Foundation, Snow Foundation, Feiock Fund, Children's Discovery Institute, Manpei Suzuki Diabetes Foundation, and a JSPS Overseas Research Fellowship.
Washington University School of Medicine's 1,500 faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children's hospitals. The School of Medicine is a leader in medical research, teaching and patient care, ranking among the top 10 medical schools in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children's hospitals, the School of Medicine is linked to BJC HealthCare.
Return to top of page.
|
|
Apr 28 2020 Fetal Timeline Maternal Timeline News
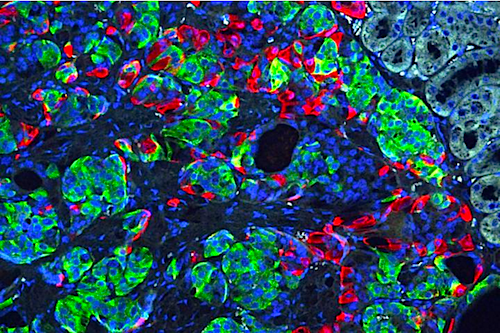 Researchers at Washington University School of Medicine in St. Louis, Missouri, have transformed stem cells into insulin-producing cells. They used the CRISPR gene-editing tool to correct a defect that caused a form of diabetes, and implanted the cells into mice to reverse diabetes in the animals. Shown is a microscopic image of insulin secreting beta cells (insulin GREEN) that were made from stem cells produced from the skin of a patient with Wolfram syndrome.
CREDIT Millman lab Washington University.
|
|



