|
|
Developmental Biology - Male Hormones
Fracking May Interrupt Male Sex Hormones
A chemical used in hydraulic fracking, has the potential to interfere with reproductive hormones in men...
A chemical used in hydraulic fracturing, commonly called fracking, has the potential to interfere with reproductive hormones in men, according to research accepted for presentation at ENDO 2020, the Endocrine Society's annual meeting, and publication in a special supplemental section of the Journal of the Endocrine Society.
The study found the chemical can block the effects of testosterone and other male sex hormones known as androgens.
"Possible adverse health outcomes associated with anti-androgen exposure are abnormal reproductive function, male infertility and disrupted testicular and prostate development," said lead researcher Phum Tachachartvanich PhD, of the University of California, Davis in Davis, Calif.
Hydraulic fracturing technology has significantly improved the yield of oil and natural gas extraction from unconventional sources. Fracking involves drilling and hydraulic extraction by injecting mixtures of industrial chemicals at high pressure into horizontal bore wells. Fracking chemicals contaminate the environment, including lake, groundwater and wastewater, and they are likely to affect everyone that is exposed to this group of chemicals, according to Tachachartvanich.
"The widespread use of fracking has led to concerns of potential negative impacts on both the environment and human health," Tachachartvanich said. "Everyone should be concerned about fracking as the wastewater generated has potential endocrine-disrupting effects, which can adversely affect the general population."
The researchers used a computer model to rank 60 hydraulic fracturing chemicals used in California, based on the predicted potential of each chemical to interfere with androgens' ability to bind with cells in the body. Based on the rankings, they used a cell model to verify the top five fracking chemicals that showed the highest potential to interfere with this process.
They then measured the androgen binding activity in the cell model for each chemical. Of the five HF chemicals tested, only one - Genapol-X100--significantly inhibited androgen binding activity. "This suggests Genapol-X100 has endocrine-disrupting abilities," Tachachartvanich said.
###
The Endocrine Society canceled its annual meeting, ENDO 2020, amid concerns about COVID-19. Visit our online newsroom for more information on accepted abstracts, which will be published in a special supplemental section of the Journal of the Endocrine Society.
Endocrinologists are at the core of solving the most pressing health problems of our time, from diabetes and obesity to infertility, bone health, and hormone-related cancers. The Endocrine Society is the world's oldest and largest organization of scientists devoted to hormone research and physicians who care for people with hormone-related conditions.
The Society has more than 18,000 members, including scientists, physicians, educators, nurses and students in 122 countries. To learn more about the Society and the field of endocrinology, visit our site at http://www.endocrine.org. Follow us on Twitter at @TheEndoSociety and @EndoMedia.
Abstract
Pluripotent stem cells are increasingly used to model different aspects of embryogenesis and organ formation1. Despite recent advances in in vitro induction of major mesodermal lineages and cell types2,3, experimental model systems that can recapitulate more complex features of human mesoderm development and patterning are largely missing. Here we used induced pluripotent stem cells for the stepwise in vitro induction of presomitic mesoderm and its derivatives to model distinct aspects of human somitogenesis. We focused initially on modelling the human segmentation clock, a major biological concept believed to underlie the rhythmic and controlled emergence of somites, which give rise to the segmental pattern of the vertebrate axial skeleton. We observed oscillatory expression of core segmentation clock genes, including HES7 and DKK1, determined the period of the human segmentation clock to be around five hours, and demonstrated the presence of dynamic travelling-wave-like gene expression in in vitro-induced human presomitic mesoderm. Furthermore, we identified and compared oscillatory genes in human and mouse presomitic mesoderm derived from pluripotent stem cells, which revealed species-specific and shared molecular components and pathways associated with the putative mouse and human segmentation clocks. Using CRISPR–Cas9-based genome editing technology, we then targeted genes for which mutations in patients with segmentation defects of the vertebrae, such as spondylocostal dysostosis, have been reported (HES7, LFNG, DLL3 and MESP2). Subsequent analysis of patient-like and patient-derived induced pluripotent stem cells revealed gene-specific alterations in oscillation, synchronization or differentiation properties. Our findings provide insights into the human segmentation clock as well as diseases associated with human axial skeletogenesis.
Authors
Mitsuhiro Matsuda, Yoshihiro Yamanaka, Maya Uemura, Mitsujiro Osawa, Megumu K. Saito, Ayako Nagahashi, Megumi Nishio, Long Guo, Shiro Ikegawa, Satoko Sakurai, Shunsuke Kihara, Thomas L. Maurissen, Michiko Nakamura, Tomoko Matsumoto, Hiroyuki Yoshitomi, Makoto Ikeya, Noriaki Kawakami, Takuya Yamamoto, Knut Woltjen, Miki Ebisuya, Junya Toguchida and Cantas Alev.
Acknowledgements
The authors thank B. McIntyre and P. O’Neill for critical reading of the manuscript; K. Mitsunaga for help with FACS analysis; Y. Ashida for help with development of human spheroid PSM-induction protocol; H. Hayashi for help with development of mouse PSM protocol; J. Asahira for help with RNA-seq experiments; A. Yamashita for help with 3D chondrogenic induction experiments; M. Shibata and T. Nakajima for help with development of one-step PSM-induction protocol; M. Ohno and S. Nishimura for help with iPS cell quality control and validation; members of the Kageyama laboratory, K. Yoshioka-Kobayashi and A. Isomura for help with Hilbert transformation and M. Matsumiya for help with removing spike noise from images; the CiRA Genome Evaluation Group, in particular H. Dohi, F. Kitaoka, M. Nomura, T. Takahashi, M. Umekage and N. Takasu for performing SNP array analysis. This work was supported by the CiRA Fellowship Program of Challenge to C.A.; Naito Foundation Research Grant to C.A.; Grant-in-Aid for Challenging Exploratory Research (KAKENHI Number 16K15664) to C.A.; Grant-in-Aid for Scientific Research on Innovative Areas (KAKENHI Number 17H05777) to M.M.; Takeda Science Foundation Grant to M.E.; Japan Agency for Medical Research and Development (AMED) Grants Number 12103610 and 17935423 to M.K.S. for iPS cell generation and qualification, grant number JP19bm0804001 to K.W. for iPS cell gene editing and grant numbers JP18ek0109212 and 18ek0109280 to S.I. for genomic and exome studies of spondylocostal dysostosis, respectively; the Core Center for iPS Cell Research (AMED) to T.Y., K.W. and J.T. and the Acceleration Program for Intractable Disease Research Using Disease Specific iPS Cells (AMED) to K.W., J.T. and M.K.S.; the Kyoto University Hakubi Project to K.W.; the Cooperative Research Program (Joint Usage/Research Center Program) of the Institute for Frontier Life and Medical Sciences, Kyoto University to J.T., L.G and S.I.. ASHBi is supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
About Kyoto University
Kyoto University is one of Japan and Asia's premier research institutions, founded in 1897 and responsible for producing numerous Nobel laureates and winners of other prestigious international prizes. A broad curriculum across the arts and sciences at both undergraduate and graduate levels is complemented by numerous research centers, as well as facilities and offices around Japan and the world. For more information please see: http://www.kyoto-u.ac.jp/en
Return to top of page.
| |
|
Apr 30 2020 Fetal Timeline Maternal Timeline News
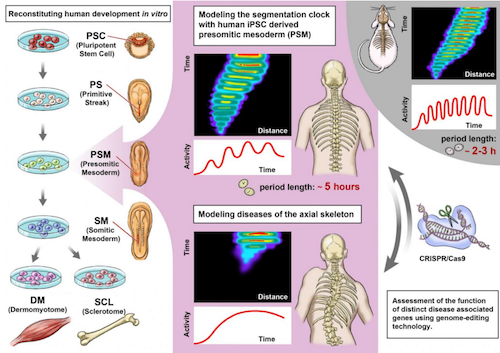 Graphical abstract of the current paper. Researchers reconstituted the human segmentation clock with iPS cells and analyzed the key genes involved. CREDIT Kyoto University: Cantas Alev & Misaki Ouchida.
|



