|
|
Developmental Biology - Gene Therapy
A Better Gene Therapy Technique?
An acoustic approach to gene therapies made specific to various cancers and genetic disorders...
A UCLA-led research team reports it has a method, first published in PNAS on April 6 of 2015, for delivering DNA into stem cells and immune cells safely, rapidly and economically. Described in the journal PNAS, Proceedings of the National Academy of Sciences the method could give scientists a new tool for manufacturing gene therapies for people with cancer, genetic disorders and blood diseases.
"We are figuring out how to get gene-editing tools into cells efficiently, safely and economically. We want to get them into enormous numbers of cells without using viruses, electroshock treatments or chemicals that will rip open the membrane and kill many of the cells, and our results so far are promising."
Paul Weiss PhD, UCLA distinguished professor, Chemistry and Biochemistry, Bioengineering and of Materials Science and Engineering, California NanoSystems Institute (CNSI), University of California, Los Angeles, California USA, and study co-senior author.
In current practice, cells used for genetic therapies are sent to specialized labs, which can take up to two months to produce an individualized treatment. Such treatments are expensive: A single regimen for one patient can cost hundreds of thousands of dollars.
"We hope our method could be used in the future to prepare treatments that can be performed at the patient's bedside," explains Weiss.
The method could be used along with CRISPR, the genetic engineering technique that enables DNA to be edited with remarkable precision. However, using CRISPR efficiently, safely and economically in medical therapies has proven to be a challenge - one this new 'acoustic' method may be able to solve.
The mechanism uses high-frequency acoustic waves coupled with millions of cells flowing through an "acoustofluidic device." Invented as part of the research, inside the device - electrical signals are converted into mechanical vibrations which can manipulate cell pores. The acoustic vibrations open pores in the cell membrane allowing DNA and other biologic cargo to pass through cell walls. This step enables researchers to insert biologic cargo without risk of damaging the cell through direct contact.
"When combined with new gene-editing approaches, this method enables us to correct a DNA sequence that is miscoded in a disease. The viability is very high compared with other techniques, but we still want higher efficiencies and are working toward that goal." explains Weiss. Plasmids entering through cell walls, are used as templates for gene editing as they have the right code sequence for a desired protein, he continued.
Steven Jonas MD, PhD, FAAP, UCLA clinical instructor in pediatrics and study co-senior author, likened the soundwaves' ability to penetrate cells to the experience of audience members actually feeling sound at a concert.
"At a concert hall, you can feel the bass - and if you can feel the sound, the cell can feel the acoustic wave. We can engineer the acoustic waves to direct the cells as needed."
Steven Jonas MD, PhD, FAAP, member of the California NanoSystems Institute, UCLA, the UCLA Jonsson Comprehensive Cancer Center and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, UCLA, USA.
Researchers delivered short strands of DNA called plasmids into human blood cells and blood-forming stem cells that were intended specifically for laboratory research, pumping millions of such cells through the acoustofluidic device. Once inside a cell, a plasmid can become a protein that may be missing or damaged, to give that cell new capabilities.
Lead author Jason Belling, a UCLA graduate student in chemistry and biochemistry, was able to insert plasmids into the model cells used for testing about 60% of the time, without using any chemical or physical treatments.
Jonas MD, whose expertise is in treating childhood cancer and blood disorders, believes the research has the potential to benefit adults and children with cancer, immune system disorders and genetic diseases. He says some existing treatments can take a patient's T cells and adapt them with a gene that encodes for a receptor that allows it to target the cancer.
"If the delivery works, and it seems to, this research is an important step toward bringing new therapies to the patients who need them. Traditionally, we have treated cancers with chemotherapy, surgery, radiation and bone marrow transplantations. Now, we're at an amazing era of medicine, where we can use different types of gene therapies that can train the immune system to fight cancer. We want to be the delivery service that gets these therapeutic packages to the cells. I want to treat my patients with cells that are engineered in this way."
Steven Jonas MD, PhD, FAAP.
For the technique to be viable treating disease, it would need to process hundreds of million cells - and in some cases, billions of cells - safely, rapidly and cost-effectively for each patient.
The approach is still the subject of research and is not available to treat human patients.
Significance
The separation and analysis of circulating tumor cells (CTCs) provides physicians a minimally invasive way to monitor the response of cancer patients to various treatments. Among the existing cell-separation methods, acoustic-based approaches provide significant potential to preserve the phenotypic and genotypic characteristics of sorted cells, owing to their safe, label-free, and contactless nature. In this work, we report the development of an acoustic-based device that successfully demonstrates the isolation of rare CTCs from the clinical blood samples of cancer patients. Our work thus provides a unique means to obtain viable and undamaged CTCs, which can subsequently be cultured. The results presented here offer unique pathways for better cancer diagnosis, prognosis, therapy monitoring, and metastasis research.
Abstract
Circulating tumor cells (CTCs) are important targets for cancer biology studies. To further elucidate the role of CTCs in cancer metastasis and prognosis, effective methods for isolating extremely rare tumor cells from peripheral blood must be developed. Acoustic-based methods, which are known to preserve the integrity, functionality, and viability of biological cells using label-free and contact-free sorting, have thus far not been successfully developed to isolate rare CTCs using clinical samples from cancer patients owing to technical constraints, insufficient throughput, and lack of long-term device stability. In this work, we demonstrate the development of an acoustic-based microfluidic device that is capable of high-throughput separation of CTCs from peripheral blood samples obtained from cancer patients. Our method uses tilted-angle standing surface acoustic waves. Parametric numerical simulations were performed to design optimum device geometry, tilt angle, and cell throughput that is more than 20 times higher than previously possible for such devices. We first validated the capability of this device by successfully separating low concentrations (~100 cells/mL) of a variety of cancer cells from cell culture lines from WBCs with a recovery rate better than 83%. We then demonstrated the isolation of CTCs in blood samples obtained from patients with breast cancer. Our acoustic-based separation method thus offers the potential to serve as an invaluable supplemental tool in cancer research, diagnostics, drug efficacy assessment, and therapeutics owing to its excellent biocompatibility, simple design, and label-free automated operation while offering the capability to isolate rare CTCs in a viable state.
Authors
Peng Li, Zhangming Mao, Zhangli Peng, Lanlan Zhou, Yuchao Chen, Po-Hsun Huang, Cristina I. Truica, Joseph J. Drabick, Wafik S. El-Deiry, Ming Dao, Subra Suresh, and Tony Jun Huang
Acknowledgements
The authors thank Mr. Joseph Rufo for manuscript editing. We gratefully acknowledge financial support from NIH Grants 1 R01 GM112048-01A1 and 1R33EB019785-01, the National Science Foundation, and the Penn State Center for Nanoscale Science (Materials Research Science and Engineering Center) under Grant DMR-0820404. Z.P. and M.D. also acknowledge partial support from NIH Grant U01HL114476.
Return to top of page.
| |
|
May 1 2020 Fetal Timeline Maternal Timeline News
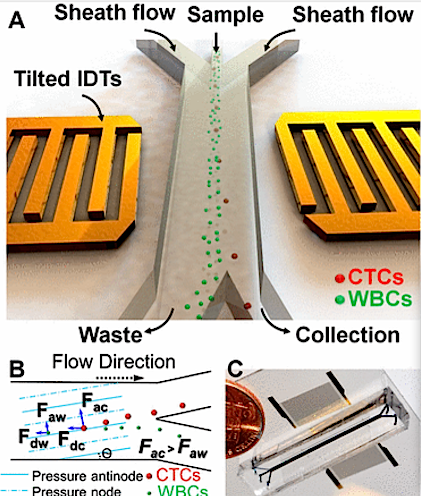 (A) Illustration of taSSAW-based cell separator. (B) Schematic of Cell Separation Machine.
Pressure within nodes and antinodes is established by the incline of each micro-channel. Drag forces experienced by Circulating Tumor Cells ( CTCs) are identified as 'Fdc'. In White Blood Cells ( WBCs), drag forces are identified as 'Fdw'. CTCs have larger vertical displacement than WBCs and produce a larger acoustic radiation force (FAC) than WBCs (FAW).
(C) The Actual taSSAW cell separation device with blue ink filling in its microfluidic channels.
|



