|
|
Developmental Biology - SARS-CoV-2 Antibody Test
A Sensitive Test for Coronavirus Antibodies
Sensitive new test detects antibodies against SARS-CoV-2 in only 10 minutes...
As the COVID-19 curve shows signs of flattening in the U.S. and elsewhere, public health officials are trying to grasp just how many people have been infected. Now, a proof-of-concept study in ACS' Analytical Chemistry describes a quick, sensitive test for antibodies against the coronavirus in human blood. The test could help doctors track a person's exposure to the disease, as well as confirm suspected COVID-19 cases that tested negative by other methods.
Because COVID-19 symptoms range from mild to severe, with some people apparently having no symptoms, the number of people who have been infected with the SARS-CoV-2 virus at some point is likely much higher than the number of confirmed cases.
As U.S. states begin to ease lockdown restrictions, widespread testing of the general population will be important to identify people at early stages of disease, or people who lack symptoms but can still infect others.
Also, although more research needs to be done, it is possible that people with antibodies to the virus could be immune to future COVID-19 outbreaks.
To help identify people with current or past exposure to SARS-CoV-2, Lei Yu, Yingsong Wu, Guanfeng Lin and colleagues wanted to develop a fast, sensitive antibody test.
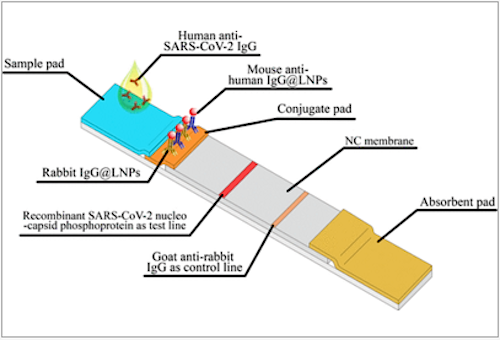
Researchers based their test on a technique called a lateral flow immunoassay (LFA); a home pregnancy test is an example of this kind of assay.
• First, researchers attached a viral coat protein to a specific region on a strip of nitrocellulose.
• They then added human serum.
• Serum flows from one end of the strip to the other.
• Any antibodies against the viral protein binds to that region of the strip.
• Any anti-SARS-CoV-2 antibodies will be fluorescently labeled.
This fluorescence-based detection is much more sensitive than some other LFAs, such as pregnancy tests, that can be read by the naked eye.
The researchers tested the new assay on seven serum samples from COVID-19 patients and 12 samples from people who had tested negative for the disease by reverse transcriptase-polymerase chain reaction (RT-PCR), a common diagnostic test that occasionally fails to detect positive cases.
The new assay correctly diagnosed all seven samples as positive - as well as an additional "negative" case that had suspicious clinical symptoms - in only 10 minutes per sample.
This immunoassay could be helpful in confirming negative diagnoses, monitoring a patient's recovery, studying past exposures, and identifying recovered individuals with high levels of antibodies as potential convalescent plasma donors, the researchers say.
The paper appears in the journal of the American Chemical Society, ACS, Publications.
Significance
The separation and analysis of circulating tumor cells (CTCs) provides physicians a minimally invasive way to monitor the response of cancer patients to various treatments. Among the existing cell-separation methods, acoustic-based approaches provide significant potential to preserve the phenotypic and genotypic characteristics of sorted cells, owing to their safe, label-free, and contactless nature. In this work, we report the development of an acoustic-based device that successfully demonstrates the isolation of rare CTCs from the clinical blood samples of cancer patients. Our work thus provides a unique means to obtain viable and undamaged CTCs, which can subsequently be cultured. The results presented here offer unique pathways for better cancer diagnosis, prognosis, therapy monitoring, and metastasis research.
Abstract
The outbreak of 2019 coronavirus disease (COVID-19) has been a challenge for hospital laboratories because of the huge number of samples that must be tested for the presence of the causative pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Simple and rapid immunodiagnostic methods are urgently needed to identify positive cases. Here we report the development of a rapid and sensitive lateral flow immunoassay (LFIA) that uses lanthanide-doped polysterene nanoparticles (LNPs) to detect anti-SARV-CoV-2 IgG in human serum. A recombinant nucleocapsid phosphoprotein of SARS-CoV-2 was dispensed onto a nitrocellulose membrane to capture specific IgG. Mouse anti-human IgG antibody was labeled with self-assembled LNPs that served as a fluorescent reporter. A 100-?l aliquot of serum samples (1:1000 dilution) was used for this assay and the whole detection process took 10 min. The results of the validation experiment met the requirements for clinical diagnostic reagents. A value of 0.0666 was defined as the cutoff value by assaying 51 normal samples. We tested 7 samples that were positive by reverse-transcription (RT-)PCR and 12 that were negative but clinically suspicious for the presence of anti-SARS-CoV-2 IgG. One of the negative samples was determined to be SARS-CoV-2 IgG positive, while the results for the other samples were consistent with those obtained by RT-PCR. Thus, this assay can achieve rapid and sensitive detection of anti-SARS-CoV-2 IgG in human serum and allow positive identification in suspicious cases; it can also be useful for monitoring the progression COVID-19 and evaluating patients’ response to treatment.
Authors
Zhenhua Chen, Zhigao Zhang, Xiangming Zhai, Yongyin Li, Li Lin, Hui Zhao, Lun Bian, Peng Li, Lei Yu, Yingsong Wu and Guanfeng Lin.
Acknowledgements
The authors acknowledge funding from the National Natural Science Foundation of China and the China Postdoctoral Science Foundation.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS' mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and its people. The Society is a global leader in providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a specialist in scientific information solutions (including SciFinder® and STN®), its CAS division powers global research, discovery and innovation. ACS' main offices are in Washington, D.C., and Columbus, Ohio.
Return to top of page.
| |
|
May 5 2020 Fetal Timeline Maternal Timeline News
A new lateral flow immunoassay can detect antibodies against SARS-CoV-2, appearing as a
BRIGHT ORANGE LINE (RIGHT) under a fluorescence reader. CREDIT Guanfeng Lin.
|



