|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - COVID-19 New Cell Crucial In Response to Respiratory Infection A study just published in Cell, is the first to show one of the mechanisms through which convalescent plasma and the virus-specific antibodies in it work — via boosting inf-cDC2. Boosted DCs induce a much stronger immune response, this study reveals a new target for therapeutic intervention for viral infections and other inflammatory diseases. With a discovery that could rewrite immunology textbooks, an international group of scientists, now play a crucial role in identifying antigens to other immune cells found during viral respiratory infections. This discovery could explain how convalescent plasma helps boost immune responses in virus-infected patients. Scientists from the VIB-UGent Center for Inflammation Research, have found a new type of antigen presenting immune cell, part of an expanding family of dendritic cells (DCs). Bart Lambrecht, Martin Guilliams, Hamida Hammad, and Charlotte Scott (all of the VIB-UGent Center for Inflammation Research) also identified antigens to other immune cells they found during respiratory virus infections. These antigens might explain how convalescent plasma helps boost immune responses in other virus-infected patients. Inflammation and Immunity When our body faces an infection, it responds with inflammation and fever. This is a sign that our immune system is working. This response activates many cells, behaving like soldiers in an immune army. Dendritic cells (DCs) are the generals in that army. They can precisely activate and instruct "soldier cells" to kill infected cells by presenting antigens created from viral "invaders" via our immune system. Mistaken identity There are several types of DCs that perform antigen-presenting functions in the body. A first type of conventional DCs continuously scan the body for dangerous invaders, even without infection. Infection triggers another subset of DCs to emerge from inflammatory monocytes. Because monocyte derived DCs are easily prepared in vitro from monocytes isolated from human blood, it was assumed they were very important antigen-presenting cells. Clinical trials using monocyte-derived DCs in cancer therapy, have been disappointing. The study reveals that monocyte-derived DCs are poor antigen-presenting cells, but only due to a case of mistaken identity. The scientists studied mice given a viral respiratory infection (mouse pneumonia and influenza viruses). Single-cell resolution then allowed them to separate monocyte-derived cells from other DCs responding to the infections. They found monocyte-derived DCs do exist, but actually do not make antigens. The reason for all the past confusion are look-alike DCs. These new DCs are now called inflammatory type 2 conventional DCs, or inf-cDC2. They appear to combine some of the best characteristics of monocytes, macrophages, and conventional DCs, and induce the best form of immunity. "This was a big surprise for us. We've all been taught that monocyte-derived cells are excellent antigen presenting cells, certainly when there's inflammation. Now, we show that it's actually a new hybrid DC type that's doing all the work. This really changes what we know about the immune system and is very important knowledge for understanding respiratory viral infections and other inflammatory diseases." Summary Highlights • Microbiota controls constitutive IFN-I production by pDCs at steady state • Tonic IFNAR signaling instructs a basal state of cDCs, poised for future immune combat • Tonic IFN-I signals license cDCs for T cell priming against harmless peripheral antigens • Reduced metabolic fitness of Ifnar1 -/- cDCs cannot be overcome by direct activation Summary Environmental signals shape host physiology and fitness. Microbiota-derived cues are required to program conventional dendritic cells (cDCs) during the steady state so that they can promptly respond and initiate adaptive immune responses when encountering pathogens. However, the molecular underpinnings of microbiota-guided instructive programs are not well understood. Here, we report that the indigenous microbiota controls constitutive production of type I interferons (IFN-I) by plasmacytoid DCs. Using genome-wide analysis of transcriptional and epigenetic regulomes of cDCs from germ-free and IFN-I receptor (IFNAR)-deficient mice, we found that tonic IFNAR signaling instructs a specific epigenomic and metabolic basal state that poises cDCs for future pathogen combat. However, such beneficial biological function comes with a trade-off. Instructed cDCs can prime T cell responses against harmless peripheral antigens when removing roadblocks of peripheral tolerance. Our data provide fresh insights into the evolutionary trade-offs that come with successful adaptation of vertebrates to their microbial environment. Authors Laura Schaupp, Sabine Muth, Leif Rogell, Michael Kofoed-Branzk, Felix Melchior, Stefan Lienenklaus, Stephanie C. Ganal-Vonarburg, Matthias Klein, Fabian Guendel, Tobias Hain, Kristian Schütze, Ulrike Grundmann, Vanessa Schmitt, Martina Dorsch, Julia Spanier, Pia-Katharina Larsen, Thomas Schwanz, Sven Jäckel, Christoph Reinhardt, Tobias Bopp, Sven Danckwardt, Karsten Mahnke, Gitta Anne Heinz, Mir-Farzin Mashregh, Pawel Durek, Ulrich Kalinke, Oliver Kretz, Tobias B. Huber, Siegfried Weiss, Christoph Wilhelm, Andrew J. Macpherson, Hansjörg Schild, Andreas Diefenbach, Hans Christian Probst. Acknowledgements This study was funded by the European Research Council, University Ghent, Research Foundation Flanders (FWO), and the Health Research Council New Zealand. Research Partners and Funding Principal partners involved in the research were Prof. Dr. Hansjörg Schild, Dr. Hans Christian Probst and Dr. Sabine Muth of the Institute for Immunology/Research Center for Immunotherapy, University Medical Center Mainz. Other key partners were Prof. Dr. Stephanie Ganal-Vonarburg and Prof. Dr. Andrew Macpherson in Bern. Dr. Mir-Farzin Mashreghi of the German Rheumatism Research Center Berlin (DRFZ) was responsible for RNA sequencing. Other important partners included Prof. Stefan Lienenklaus and Prof. Dr. Ulrich Kalinke of the Hanover Medical School (MHH). Epigenome analyses were performed in collaboration with Dr. Thomas Manke of the Max Planck Institute of Immunobiology and Epigenetics in Freiburg. Metabolic analyses were performed in collaboration with Dr. Christoph Wilhelm of the Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital Bonn. The study received substantial funding from the European Research Council (A. Diefenbach) and the German Research Foundation (A. Diefenbach, H.C. Probst and H. Schild). Return to top of page. | May 11 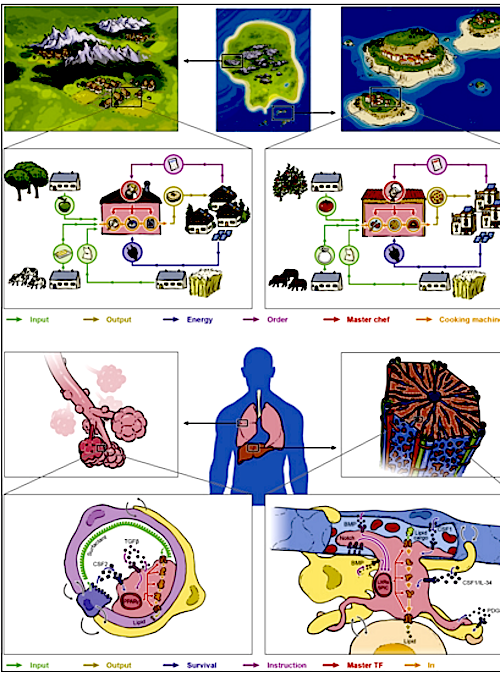 Macrophages in tissue modules act like isolated villages, whether located in the lung or the liver. Like the village BAKERY (left) or PIZZERIA (right) there are complex interactions. CREDIT Natan Shaked/AFTAU.
|
||||||||||||||||||||||||||||

