|
|
Developmental Biology - COVID-19
YAP Protein Can Promote Cancers
Cell-to-cell contact should slow cancer cell proliferation, but cancer skips this message...
Like subway com muters on a crowded train, cells generally prefer not to be packed in too tightly. In fact, they have set up mechanisms to avoid this, a phenomenon called "contact inhibition." A hallmark of cancer cells is that they lack this contact inhibition, and instead become pushy, facilitating their spread.
Scientific understanding of the mechanism underlying this cell behavior change has had many gaps. A new paper from the lab of Joseph Kissil, PhD, professor of Molecular Medicine at Scripps Research in Florida, provides important new insights.
Writing in Cancer Research, a journal of the American Association for Cancer Research, Kissil and colleagues offer new details about how the "stop-growth" signal unfurls during cell-to-cell contact, and how disruption of that "stop-growth" signal can promote cancer.
A key player is a protein called YAP, a regulator of gene expression. YAP is a major effector of a pathway referred to as Hippo, after a mutation discovered in the HPO gene. It produces lumpy, hippo-like tissue overgrowth in fruits.
Healthy cells and developing organs "know" when they should grow and when they should stop growing, based on multiple signaling molecules. These signals are transmitted by YAP and the Hippo pathway. Tracing out those signals is not only central to understanding our basic biology, but to finding new ways to attack cancers with precision therapies, Kissil explains.
Increasing cell density normally activates a change in cell signaling. It does this via an elevation of protein involved in initiating contact inhibition, p27. But a disrupted Hippo pathway interferes with normal YAP behavior and blocks the expected p27 surge.
Kissil says the team was surprised to find YAP in the uncharacteristic role of shutting down gene transcription. Previous studies suggested that YAP is an activator of genes that promote cell growth. The reality proved to be much more complex.
"What we show here is that YAP can also turn off genes, not just turn them on," Kissil says. "It shuts down genes that would otherwise prevent cells from proliferating."
In the end, like a broken down subway line, disruption of the Hippo pathway at any point can redirect the cell's behavior, away from contact inhibition. The discovery of YAP's dual role as both promotor and repressor of gene transcription provides important information in the efforts to make cancer drugs that act on YAP.
"When we target YAP in cancer, we are targeting its function as an activator of cancer, but we now know we also need to consider its suppressive functions, as well," Kissil said. "We have to consider both the activation and the repression."
Finding the players that both interact with YAP and have a functional role in promoting cancer growth required use of a genome-wide bioinformatics technique called ChIP-seq. The work involved collaboration with the labs of Matthew Pipkin, PhD, of Scripps Research in Florida, and Michael Kareta, PhD, of Sanford Research in Sioux Falls, South Dakota. They used ChIP-seq to map the overlapping relationships of the YAP-linked genes, which number in the thousands.
They worked specifically in human Schwann cells, which are peripheral nerve cells that produce the insulating myelin around nerves, but the findings should apply to other cancers, Kissil says.
The team looked at YAP in the context of cell crowding. They learned that YAP's role involves recruitment of other interacting proteins that include YY1, also known as Yin-Yang 1, EZH2 and a protein complex called PRC2. Those will also be important to study further, Kissel says, as well as the interaction of these players in the context of cancer drug resistance.
Abstract Highlights
The Hippo pathway regulates cell proliferation and organ size through control of the transcriptional regulators YAP (yes-associated protein) and TAZ. Upon extracellular stimuli such as cell-cell contact, the pathway negatively regulates YAP through cytoplasmic sequestration. Under conditions of low cell density, YAP is nuclear and associates with enhancer regions and gene promoters. YAP is mainly described as a transcriptional activator of genes involved in cell proliferation and survival. Using a genome-wide approach, we show here that, in addition to its known function as a transcriptional activator, YAP functions as a transcriptional repressor by interacting with the multifunctional transcription factor Yin Yang 1 (YY1) and Polycomb repressive complex (PRC2) member EZH2. YAP co-localized with YY1 and EZH2 on the genome to transcriptionally repress a broad network of genes mediating a host of cellular functions, including repression of the cell-cycle kinase inhibitor p27, whose role is to functionally promote contact inhibition. This work unveils a broad and underappreciated aspect of YAP nuclear function as a transcriptional repressor and highlights how loss of contact inhibition in cancer is mediated in part through YAP repressive function.
Authors
Sany Hoxha, Alyssa Shepard, Scott Troutman, Huitian Diao, Joanne R. Doherty, Michalina Janiszewska, Robert M Witwicki, Matthew E Pipkin, William W Ja, Michael S Kareta and Joseph L. Kissil
Acknowledgements
About Scripps Research
Scripps Research is an independent, nonprofit biomedical institute ranked the most influential in the world for its impact on innovation. With campuses in La Jolla, California, and Jupiter, Florida, we are advancing human health through profound discoveries that address pressing medical concerns around the globe. Our drug discovery and development division, Calibr, works hand-in-hand with scientists across disciplines to bring new medicines to patients as quickly and efficiently as possible, while teams at Scripps Research Translational Institute harness genomics, digital medicine and cutting-edge informatics to understand individual health and render more effective healthcare. Scripps Research also trains the next generation of leading scientists at our Skaggs Graduate School, consistently named among the top 10 U.S. programs for chemistry and biological sciences. Learn more at http://www.scripps.edu.
Return to top of page.
| |
|
May 19 2020 Fetal Timeline Maternal Timeline News
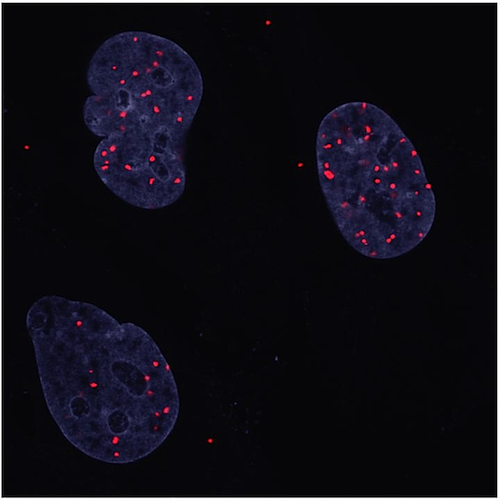 Cells prefer not to be crowded and stop proliferating if too densely packed. Here you see the interaction between two proteins (YAP and YY1) in the nuclei of human Schwann cells. Working together, they can shut off cell division. RED dots represent their interaction, nuclei are stained BLUE.
CREDIT Kissil Lab at Scripps Research
|



