|
|
Developmental Biology - COVID19 Vascular Damage
COVID Deaths Show Clotting in Blood Vessels
COVID-19 damages the endothelial cells lining blood vessels, causing severe injury with oxygenation of blood...
In a new study in the New England Journal of Medicine (NEJM), senior author, Steven J. Mentzer, MD, thoracic surgeon at Brigham and Women's Hospital, and a team of international researchers examined seven lungs obtained during autopsy from patients who died of COVID-19.
They compared this group to seven autopsied lungs obtained from patients who died of acute respiratory distress syndrome secondary to influenza A (H1N1) infection as well as to 10 age-matched uninfected control lungs.
Both COVID-19 and influenza are the same category of virus and infect the respiratory tract.
While the lungs shared some common features, there are distinct features related to blood vessels seen in patients who had died of COVID-19.
Researchers observed that COVID-19 damaged the endothelial cells (vascular lining cells), causing severe endothelial injury.
Patients with COVID-19 showed widespread blood clotting as well as new vessel growth, likely a result of the body's response to the virus.
They saw signs of distinct pulmonary vascular disease progressing in some cases of COVID-19 as compared to equally severe influenza virus infection.
Some Key Points
• COVID-19 is a respiratory virus that causes
vascular [blood vessel] disease.
• Damage to vascular cells helps explain serious blood clotting seen in patients.
• A unique response, intussusceptive angiogenesis (IA), is how the body compensates for thrombosis [blood clot within a blood vessel] and its damage.
• Damaged blood vessels may underlie COVID toe, children with Kawasaki, stroke, and other "unrelated" problems seen with COVID-19.
• This study supports a need for more research on angiogenesis and the vascular effects of COVID-19.
Abstract
Background
Progressive respiratory failure is the primary cause of death in the coronavirus disease 2019 (Covid-19) pandemic. Despite widespread interest in the pathophysiology of the disease, relatively little is known about the associated morphologic and molecular changes in the peripheral lung of patients who die from Covid-19.
Methods
We examined 7 lungs obtained during autopsy from patients who died from Covid-19 and compared them with 7 lungs obtained during autopsy from patients who died from acute respiratory distress syndrome (ARDS) secondary to influenza A(H1N1) infection and 10 age-matched, uninfected control lungs. The lungs were studied with the use of seven-color immunohistochemical analysis, micro–computed tomographic imaging, scanning electron microscopy, corrosion casting, and direct multiplexed measurement of gene expression.
Results
In patients who died from Covid-19–associated or influenza-associated respiratory failure, the histologic pattern in the peripheral lung was diffuse alveolar damage with perivascular T-cell infiltration. The lungs from patients with Covid-19 also showed distinctive vascular features, consisting of severe endothelial injury associated with the presence of intracellular virus and disrupted cell membranes. Histologic analysis of pulmonary vessels in patients with Covid-19 showed widespread thrombosis with microangiopathy. Alveolar capillary microthrombi were 9 times as prevalent in patients with Covid-19 as in patients with influenza (P<0.001). In lungs from patients with Covid-19, the amount of new vessel growth — predominantly through a mechanism of intussusceptive angiogenesis — was 2.7 times as high as that in the lungs from patients with influenza (P<0.001).
Conclusions
In patients who died from Covid-19–associated or influenza-associated respiratory failure, the histologic pattern in the peripheral lung was diffuse alveolar damage with perivascular T-cell infiltration. The lungs from patients with Covid-19 also showed distinctive vascular features, consisting of severe endothelial injury associated with the presence of intracellular virus and disrupted cell membranes. Histologic analysis of pulmonary vessels in patients with Covid-19 showed widespread thrombosis with microangiopathy. Alveolar capillary microthrombi were 9 times as prevalent in patients with Covid-19 as in patients with influenza (P<0.001). In lungs from patients with Covid-19, the amount of new vessel growth — predominantly through a mechanism of intussusceptive angiogenesis — was 2.7 times as high as that in the lungs from patients with influenza (P<0.001).
Authors
Maximilian Ackermann MD, Stijn E. Verleden PhD,, Mark Kuehnel PhD, Axel Haverich MD, Tobias Welte MD, Florian Laenger MD, Arno Vanstapel PhD, Christopher Werlein MD, Helge Stark PhD, Alexandar Tzankov MD, William W. Li MD, Vincent W. Li MD, Steven J. Mentzer MD, and Danny Jonigk MD.
Acknowledgements
The authors thank Dr Kruti Maniar (Northwestern Department of Pathology), staff in the Obstetric-COVID unit at Prentice Women’s Hospital, and the Northwestern Memorial Hospital Department of Pathology.
Patients were tracked using REDCap supported by NIH UL1TR001422.
Return to top of page.
| |
|
May 26 2020 Fetal Timeline Maternal Timeline News
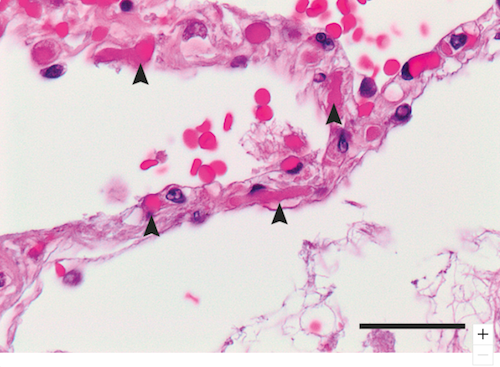 Image shows the slightly expanded alveolar walls (arrows) in the lung capillaries of Covid19 patients who have died with these blood clots. CREDIT — The Authors.
|



