|
|
Developmental Biology - Brain Development
Model For Early Human Embryonic Brain Developed
A developmental tree of the human brain is now available as a guide to different types of nerve cells for stem cell research and therapy...
We know a lot about the human brain, but very little about how it is formed. In particular, the stages from the second to the seventh week of embryonic development have so far been virtually unknown territory to brain researchers.
To learn more about this particular period, researchers from the Department of Neuroscience and the Novo Nordisk Foundation Center for Stem Cell Biology at the Faculty of Health and Medical Sciences have now developed a model that mimics these early stages of the human brain in the laboratory.
The model is based on embryonic stem cells grown in a microfluidic system developed in collaboration with bioengineers from Lund University in Sweden. Their research is published in the journal: Nature Biotechnology.
"We know that in the early embryonic stage the brain is exposed to various concentrations of growth factors which induces the formation of different brain regions. By using microfluidic methods, we can - under extremely controlled conditions - recreate the environment found in the early embryo", explains the first author on the study, Assistant Professor Pedro Rifes.
"When we expose stem cells to the controlled environment, we can create a tissue that resembles an embryonic brain at a very early stage, about 4-5 weeks after fertilisation of the egg - a stage that we have so far not been able to study."
The Developmental Tree of the Human Brain
The researchers will use the new model to make a map of the development of the brain cells - a kind of 'Developmental tree' of the brain, thereby learning new things about how the enormous complexity of different nerve cells in the human brain is formed during the early embryonic stages.´
"For the first time, we have access to a tissue that resembles the early embryonic brain, and this allows us to go in and analyse what happens to each individual cell at each stage of development," says the principal scientist behind the study, Associate Professor Agnete Kirkeby.
The idea is that brain researchers around the world will be able to use this Developmental tree of the brain as a guide to produce different types of nerve cells for stem cell therapy. By studying the natural development of the nerve cells, the researchers will be able to speed up the creation of recipes for producing specific nerve cells in the laboratory.
A Recipe for Stem Cell Treatment
Agnete Kirkeby is well aware of the importance of a faster path to stem cell treatments. Together with colleagues from Lund and Cambridge, she has for several years worked on developing a stem cell therapy for Parkinson's disease. This project required Kirkeby and her colleagues to produce a very specific type of nerve cells, the dopaminergic nerve cells, which are the cells that are lost in Parkinson's Disease.
"We have come a long way in the project and will soon be able to test the stem cell treatment in humans for the first time. But it took us more than 10 years to get this far because we depended on a trial-and-error methodology to develop the right nerve cells from the stem cells."
With knowledge from the new model, the researchers expect to be able to considerably shorten this process in the future.
"If we understand exactly how the brain develops in the early stages, we will become better at guiding the stem cells in the right direction when producing human nerve cells in the lab. This will allow us to more quickly and efficiently develop cell treatments for neurological diseases such as epilepsy, Parkinson's Disease and certain types of dementia," says Agnete Kirkeby.
New Options for testing Environmental Toxins
In addition to increasing our knowledge on brain development and easing the path to future stem cell treatments, Agnete Kirkeby believes that the embryonic brain model may serve other useful purposes as well.
"The model may be used to investigate how brain cells in the early embryonic stages react to certain chemicals surrounding us in our daily lives - these might be substances in our environment, in consumer products or in the medications that some pregnant women may require. So far, we have not had a good model to test precisely this."
Abstract
The study of brain development in humans is limited by the lack of tissue samples and suitable in vitro models. Here, we model early human neural tube development using human embryonic stem cells cultured in a microfluidic device. The approach, named microfluidic-controlled stem cell regionalization (MiSTR), exposes pluripotent stem cells to signaling gradients that mimic developmental patterning. Using a WNT-activating gradient, we generated a neural tissue exhibiting progressive caudalization from forebrain to midbrain to hindbrain, including formation of isthmic organizer characteristics. Single-cell transcriptomics revealed that rostro-caudal organization was already established at 24 h of differentiation, and that the first markers of a neural-specific transcription program emerged in the rostral cells at 48 h. The transcriptomic hallmarks of rostro-caudal organization recapitulated gene expression patterns of the early rostro-caudal neural plate in mouse embryos. Thus, MiSTR will facilitate research on the factors and processes underlying rostro-caudal neural tube patterning.
Authors
Pedro Rifes, Marc Isaksson, Gaurav Singh Rathore, Patrick Aldrin-Kirk, Oliver Knights Møller, Guido Barzaghi, Julie Lee, Kristoffer Lihme Egerod, Dylan M. Rausch, Malin Parmar, Tune H. Pers, Thomas Laurell and Agnete Kirkeby.
Acknowledgements
This study was supported by the Novo Nordisk Foundation (grant no. NNF18OC0030286 to A.K.), The Lundbeck Foundation (grant no. R190-2014-3904 to T.H.P.) and the following grants to A.K.: Innovation Fund Denmark (no. BrainStem 4108-00008 A), the Strong Research Environment at Lund University Multipark, the Swedish Research Council (no. 70862601/Bagadilico), The Crafoord Foundation, The Segerfalk Foundation, The Tore Nilsson Foundation, The Sven-Olof Janson Foundation and the Swedish Fund for Research Without Animal Experiments. The research leading to these results has received funding from the New York Stem Cell Foundation (M.P.), the European Research Council under the ERC Grant Agreement no. 30971 (M.P.), the Swedish Research Council (grant agreement no. 521-2012-5624, M.P.). The Novo Nordisk Foundation Center for Stem Cell Biology (DanStem) and the Novo Nordisk Foundation Center for Basic Metabolic Research (CBMR) are supported by Novo Nordisk Foundation grants (nos. NNF17CC0027852 and NNF18CC0034900, respectively). M.P. is a New York Stem Cell Foundation Robertson Investigator. We thank S. da Rocha Baez, I. Nilsson, M. Madrona, M. Heide Ankjær, H.K. Lilja-Fischer (CBMR Single-cell Omics Platform), H. Neil (DanStem Genomics Platform), J. Bulkescher (DanStem Imaging Platform) and A. Meligkova (DanStem Stem Cell Culture Platform) for excellent technical and bioinformatics assistance and for use of instruments.
Return to top of page.
| |
|
May 28 2020 Fetal Timeline Maternal Timeline News
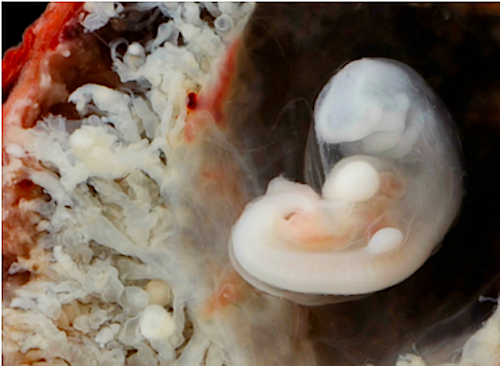 Image of a human embryo surrounded by placenta, around 7 weeks of age. Studying brain development of humans at early stages is nearly impossible, and researchers have therefore produced a model to mimic human brain developmet in the lab. CREDIT Dr Steven O'Connor (Houston, Texas).
|



