|
|
Developmental Biology - Anesthesia Effect
Century-Old Debate Solved
Anesthesia acts like a billiard ball break shot - it scatters cell structures and triggers loss of consciousness...
Now, it's effect on consciousness appears to be solved, settling a century-old scientific debate about the brain under its affect. Surgery would be inconceivable without general anesthesia, so it may come as a surprise that despite its 175-year history of medical use, doctors and scientists have been unable to explain how anesthetics temporarily render patients unconscious.
Now a study from Scripps Research published in the Proceedings of the National Academies of Sciences (PNAS) solves the longstanding mystery.
Clever experiments in cells of fruit flies, reveals that clusters of lipids within brain cell membranes move in and out of their ordered state after exposure to anesthesia. These fluctuations disturb cell signalls and cause loss of consciousness.
The discovery by chemist Richard Lerner MD, and molecular biologist Scott Hansen PhD, settles a century-old debate, still simmering today: Does anesthesia act directly on cell-membrane gates, called 'ion channels', or do anesthetics act on the membrane to signal cell changes in a new and unexpected way? It has taken the duo nearly five years of experiments to conclude that anesthesia begins a two-step process in the cell membrane:
• Anesthetics disturb order in the lipid clusters within a cell membrane — known as "lipid rafts"
• Disturbing lipid raft order - disturbs cell signals.
"We think there is little doubt this novel pathway is being used for other brain functions beyond consciousness, enabling us to now chip away at additional mysteries of the brain."
Richard Lerner MD, Member, National Academy of Sciences; Former President, Scripps Research; founder, Scripps Research Florida campus.
The Ether Dome
Ether first induced loss of consciousness in a patient in 1846 at the surgical theater of Massachusetts General Hospital, Boston. The theatre later became known as "the Ether Dome." By 1899, German pharmacologist Hans Horst Meyer, and in 1901 British biologist Charles Ernest Overton, concluded that the solubility of lipids in the cell membrane was what dictated the potency of an anesthetic.
While drafting a grant submission, Hansen did a Google search on the historic question and found a reference from Lerner's 1997 PNAS paper: "A hypothesis about the endogenous analogue of general anesthesia," proposing the role of lipid rafts' in the cell membrane.
Hansen had long looked up to Lerner - literally. As a predoctoral student at Scripps Research Center in San Diego, Hansen worked in a basement lab with a window looking directly out at Lerner's parking space.
An Exciting Moment
Hansen recalls: "I contacted him, and said; 'You are never going to believe this. Your 1997 figure was intuitively describing what I am seeing in our data right now!"
"This is the granddaddy of medical mysteries," Lerner explains. "When I was in medical school at Stanford, this was the one problem I wanted to solve. Anesthesia was of such practical importance I couldn't believe we didn't know how all of these anesthetics could cause people to lose consciousness."
Many other scientists, through a century of experimentation, had sought the same answers, but Hansen explains how they lacked several key elements:
• First, microscopes able to visualize biological complexes smaller than the limits of diffracted light.
• Second, recent insight on cell membranes: the complex organization and function of their rich mixture of lipid complexes.
"They had been looking in a whole sea of lipids, and the signal got washed out, they just didn't see it, in large part for a lack of technology," explains Hansen.
From Order to Disorder
Using Nobel Prize-winning technology in a microscope called dSTORM, short for "Direct Stochastical Optical Reconstruction Microscopy," Hansen's lab bathed cells in chloroform and then watched what happened.
Exposing the cells to chloroform increased the diameter and area of cell membrane lipid clusters known as GM1.
He watched a shift occur in GM1 cluster organization, a shift from being a tightly packed ball to becomming a disrupted mess.
As each cluster became disordered, GM1 spilled its contents, including the enzyme PLD2 (phospholipase D2).
Hansen had tagged PLD2 with a fluorescent chemical, and using the dSTORM microscope saw PLD2 shoot like a billiard ball away from its GM1 home and over to a different, less preferred lipid cluster called PIP2.
The impact activated key molecules within PIP2 clusters: TREK1 potassium ion channels and the lipid activator phosphatidic acid (PA). Activating TREK1 basically froze a neurons' ability to fire, leading to loss of consciousness, explains Hansen.
"TREK1 potassium channels release potassium, and that hyper-polarizes the nerve — making it more difficult to fire. Just shuts it down."
Scott B. Hansen PhD, Department of Molecular Medicine and Department of Neuroscience, The Scripps Research Institute, Jupiter, Florida, USA.
Lerner insisted they validate these findings in a living animal model and the common fruit fly, drosophila melanogaster, confirmed the data. Deleting PLD2 expression in the flies rendered them resistant to the effects of sedation. In fact, they required double the exposure to the anesthetic to demonstrate the same response.
"All flies eventually lost consciousness, suggesting PLD helps set a threshold. But, it is not the only pathway controlling anesthetic sensitivity," they write.
Hansen and Lerner say the discoveries raise a host of tantalizing new possibilities that may explain other mysteries of the brain, including the molecular events that lead us to fall asleep.
Lerner's original 1997 hypothesis of the role of "lipid matrices" in signaling arose from his inquiries into the biochemistry of sleep, and his discovery of a soporific lipid he called oleamide.
"We think this is fundamental and foundational. But, there is a lot more work that needs to be done - and it needs to be done by a lot of people."
Scott B. Hansen PhD
"People will begin to study this for everything you can imagine: Sleep, consciousness, all those related disorders. Ether was a gift that helps us understand the problem of consciousness. It has shined a light on a heretofore unrecognized pathway that the brain has clearly evolved to control higher-order functions."
Richard Lerner MD
Significance
Anesthetics are used every day in thousands of hospitals to induce loss of consciousness, yet scientists and the doctors who administer these compounds lack a molecular understanding for their action. The chemical properties of anesthetics suggest that they could target the plasma membrane. Here the authors show anesthetics directly target a subset of plasma membrane lipids to activate an ion channel in a two-step mechanism. Applying the mechanism, the authors mutate a fruit fly to be less sensitive to anesthetics and convert a nonanesthetic-sensitive channel into a sensitive one. These findings suggest a membrane-mediated mechanism will be an important consideration for other proteins of which direct binding of anesthetic has yet to explain conserved sensitivity to chemically diverse anesthetics.
Abstract
Inhaled anesthetics are a chemically diverse collection of hydrophobic molecules that robustly activate TWIK-related K+ channels (TREK-1) and reversibly induce loss of consciousness. For 100 y, anesthetics were speculated to target cellular membranes, yet no plausible mechanism emerged to explain a membrane effect on ion channels. Here we show that inhaled anesthetics (chloroform and isoflurane) activate TREK-1 through disruption of phospholipase D2 (PLD2) localization to lipid rafts and subsequent production of signaling lipid phosphatidic acid (PA). Catalytically dead PLD2 robustly blocks anesthetic TREK-1 currents in whole-cell patch-clamp recordings. Localization of PLD2 renders the TRAAK channel sensitive, a channel that is otherwise anesthetic insensitive. General anesthetics, such as chloroform, isoflurane, diethyl ether, xenon, and propofol, disrupt lipid rafts and activate PLD2. In the whole brain of flies, anesthesia disrupts rafts and PLDnull flies resist anesthesia. Our results establish a membrane-mediated target of inhaled anesthesia and suggest PA helps set thresholds of anesthetic sensitivity in vivo.
Authors
Mahmud Arif Pavel, E. Nicholas Petersen, ProfileHao Wang, Richard A. Lerner and Scott B. Hansen.
Acknowledgements
The authors thank Andrew S. Hansen for assisting with experimental design and discussion and comments on the manuscript, Manasa Gudheti (Vutara) for help with dSTORM data processing, Michael Frohman for mPLD2 cDNA, Guillaume Sandoz for chimeric TRAAK cDNAs, Bill Ja for help with fly experiments, and Stuart Forman for helpful discussion. This work was supported by a Director’s New Innovator Award (1DP2NS087943-01 to S.B.H.), an R01 (1R01NS112534 to S.B.H.) from the NIH, a JPB Foundation Grant (1097 to R.A.L.), and a graduate fellowship from the Joseph B. Scheller and Rita P. Scheller Charitable Foundation to E.N.P. We are grateful to the Iris and Junming Le Foundation for funds to purchase a super-resolution microscope, making this study possible.
The authors declare no competing interest.
Return to top of page.
| |
|
Jun 3 2020 Fetal Timeline Maternal Timeline News
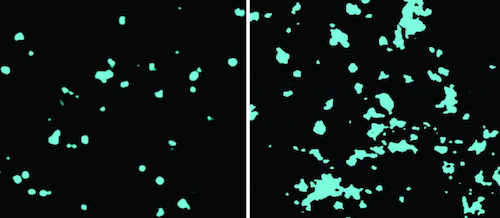 LEFT Ordered cholesterol cluster in cell membrane. RIGHT Disordered cholesterol due to chloroform. CREDIT Hansen lab, Scripps Research.
|




