|
|
Developmental Biology - Cell Structure
Taking A Deep Look Into Animal Nervous Systems
An elegant technique renders opaque tissues transparent...
A joint venture between the University of Vienna, the Medical University of Vienna, and the TU Wien (Vienna) allowed researchers to look deep into organ systems of animals from worms to fish to salamanders.
Analysis of individual cell structure and function in whole organs or tissues, is becoming increasingly important in biology.
The standard approach to microscopic investigation has been to cut tissue or organ into thin layers, examine each section, and then repbuild those layers back into a 3D model. A laborious process, which often yields incomplete results. For example, cells that make up our nervous systems, neurons, have long extensions reaching (as needed) throughout the entire body — such as from a single toe back to the brain. Reconstructing these projections from small slices is extremely challenging.
An elegant solution to avoid this is provided by tissue clearing - techniques that can render opaque tissues transparent.
When applied to complex tissues, including the brain, such techniques allow visualization of individual cells and their extensions, enabling scientists to capture 3D images of cells and tissues without the need of sectioning.
However, existing clearing techniques were not optimized to remove a variety of pigments present in tissues, limiting how deeply specimens could be imaged. So, research has essentially remained restricted to specific unpigmented organs like the brain, and a handful of species with reduced pigmentation.
Through team effort, researchers from the Max Perutz Labs [a molecular biology research center operated jointly by the University of Vienna and the Medical University of Vienna], the Medical University of Vienna and the TU Wien (Vienna University of Technology) and collaborators, developed a new method combining tissue clearing which removes numerous pigment types characteristic for most animals.
Their approach is called "DEEP-Clear". A toolkit for imaging nervous system biomolecules in a broad panel of species
Research results are published in the international journal Science Advances. An important technique that helped develop this new method was combining different chemical treatments which had a synergistic effect, allowing fast depigmentation and tissue clearing.
"Shortening chemical processing preserves the integrity of tissues and organisms, so that molecules and internal structures of interest are more likely to be retained."
Marko Pende PhD, Department for Bioelectronics, FKE, Vienna University of Technology, Vienna, Austria;
Section for Bioelectronics, Center for Brain Research, Medical University of Vienna, Vienna, Austria; and developer of the clearing method, from the lab of Hans-Ulrich Dodt.
Multiple organisms can now be imaged from different clades: mollusks and bony fish to amphibians.
"These are just a few examples. We believe that the method is applicable to multiple organisms. It was just not tried yet."
Hans Ulrich Dodt PhD, Department for Bioelectronics, FKE, Vienna University of Technology, Austria, and senior author of the study.
The team systematically explored processed samples to find which types of molecules could still be marked and detected by DEEP Clear, species ranging from squids and worms to fish and salamanders. This work - largely performed by PhD student Karim Vadiwala in the lab of Florian Raible at the Max Perutz labs - showed DEEP-Clear compatible with a variety of important biomolecules, allowing imaging of specific proteins, DNA markers and RNA in intact specimens.
"This versatility of DEEP Clear makes it a highly attractive tool to explore a range of animals for which standard tissue clearing techniques currently would not be sufficient."
Karim Vadiwala PhD, Max Perutz Labs - Research Platform “Rhythms of Life”, University of Vienna, Vienna BioCenter, Vienna, Austria.
3D Views of Whole Animal Nervous Systems
Besides its compatibility across many species, another attractive feature of DEEP-Clear is that transparency allows imaging of samples of every scale. On one hand, the team looked at minute details of contact points between neurons, and then at clusters of dividing cells.
On the other hand, they took advantage of the latest generation of so-called light-sheet microscopes developed by the Dodt lab, to rapidly scan a whole sample using two dimensional laser light. Th result is a full three-dimensional model created on computer.
"Using a very thin light-sheet enables us to overcome a lot of optical limitations and allows us to generate high-resolution images, even from samples several millimeters thick." explains Dr. Saiedeh Saghafi, TU Wien, designer of the microscopes.
The expectation of the team is that "DEEP-Clear" will popularize tissue clearing, allowing researchers around the world to intensify molecule & cell study in a variety of highly interesting species, though poorly explored. These include worms, fish, and salamanders that can regenerate parts of their central nervous system — suggesting they possess molecular capacities humans and other mammals have lost.
"Visualizing the responsible stem cells, and investigating their molecular make-up, or their contribution to regenerated tissue, will be greatly facilitated by DEEP-Clear."
Florian Raible PhD, Max Perutz Labs, and study coordinator. Raible has additional research with groups led by Dr. Oleg Simakov (Uni Vienna) and Dr. Elly Tanaka (Institute for Molecular Pathology).
Abstract
Tissue clearing combined with deep imaging has emerged as a powerful alternative to classical histological techniques. Whereas current techniques have been optimized for imaging selected nonpigmented organs such as the mammalian brain, natural pigmentation remains challenging for most other biological specimens of larger volume. We have developed a fast DEpigmEntation-Plus-Clearing method (DEEP-Clear) that is easily incorporated in existing workflows and combines whole system labeling with a spectrum of detection techniques, ranging from immunohistochemistry to RNA in situ hybridization, labeling of proliferative cells (EdU labeling) and visualization of transgenic markers. With light-sheet imaging of whole animals and detailed confocal studies on pigmented organs, we provide unprecedented insight into eyes, whole nervous systems, and subcellular structures in animal models ranging from worms and squids to axolotls and zebrafish. DEEP-Clear thus paves the way for the exploration of species-rich clades and developmental stages that are largely inaccessible by regular imaging approaches.
Authors
Marko Pende, Karim Vadiwala, Hannah Schmidbaur, Alexander W. Stockinger, Prayag Murawala, Saiedeh Saghafi, Marcus P. S. Dekens, Klaus Becker, Roger Revilla-i-Domingo, Sofia-Christina Papadopoulos, Martin Zur, Pawel Pasierbek, Oleg Simakov, Elly M. Tanaka, Florian Raible and Hans-Ulrich Dodt.
Acknowledgements
DEEP-Clear protocol and its combination with other techniques
Details on cloning, the detailed DEEP-Clear protocol in different clades, the combination of DEEP-Clear with immunostaining, and the combination of DEEP-Clear with RNA in situ hybridization and EdU labeling are listed in Supplementary Material and Methods.
Return to top of page.
| |
|
Jun 4 2020 Fetal Timeline Maternal Timeline News
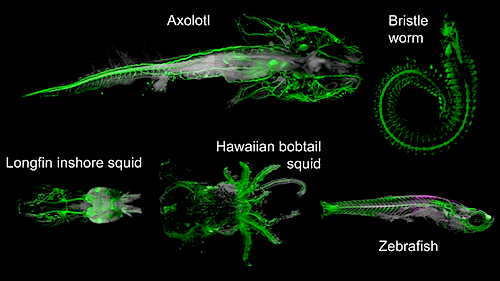 Light-sheet images of different DEEP-Clear processed animals - with neurosystems labelled with specific markers.
CREDIT TU Wien/Max Perutz Labs.
|



