|
|
Developmental Biology - Cancer and Viruses
Inflammation Protects Cancer Cells from Viruses
Francis Crick Institute uncovers how cancer cells protect themselves from viruses harmful to tumours...
Researchers at Francis Crick Institute in the United Kingdom (UK) have uncovered how cancer cells protect themselves from viruses harmful to tumours but not to healthy cells. The findings could lead to improved viral treatments for some diseases.
In their study, published in Nature Cell Biology, researchers identified a mechanism protecting cancer cells from oncolytic viruses that prefer to infect and kill cancer cells.
These viruses are sometimes used to destroy cancer cells and stimulate an immune response against a tumour. However, they only work in a minority of patients and whether they are effective or not, are not yet fully understood.
The team examined the environment surrounding a tumour and how cancer cells interact with their neighbours, in particular, cancer-associated fibroblasts (CAFs), which researchers know play a significant role in cancer protection, growth and spread.
They found when cancer cells are in direct contact with CAFs, inflammation occurs that alerts the surrounding tissue, making it harder for viruses to invade and replicate within the cancer cell.
This protective inflammatory response occurs when cancer cells pass small amounts of cytoplasm, the fluid within their cells, through to the CAFs. This triggers fibroblasts to signal nearby cells to release cytokines — molecules that cause inflammation.*
*The transfer of cytoplasm from cancer cells into fibroblasts leads to the activation of STING and IRF3-mediated expression of interferon-?1 and other cytokines.
"This process only occurs when cancer cells and fibroblasts are in direct contact with each other. In healthy tissue, this type of inflammatory response would only happen during injury, as there is usually a membrane keeping them apart."
"This is an excellent example of the way cancer hijacks our body's protective mechanisms for its own gain."
Erik Sahai PhD, Director, Tumour Cell Biology Laboratory, Crick Institute, London, UK and paper author.
Importantly, when researchers blocked the signalling pathway in tumours grown in laboratory cell cultures, cancer cells became more sensitive to oncolytic viruses. Researchers hope in the future these findings may help develop a treatment to modulate inflammation — and help oncolytic viruses to more effectively target cancer cells.
"If we can fully understand how cancer cells protect themselves from oncolytic viruses — and effective ways to stop these protective mechanisms, viruses could become a more powerful tool doctors use to treat cancer. This research is an important, early step."
Emma Milford PhD candidate and co-lead author, Tumour Cell Biology Laboratory, Francis Crick Institute, London, United Kingdom.
"These viruses prefer to target cancer cells over healthy cells, which has made them become of interest to scientists over the last few decades. However, much more remains to be understood about how they interact with tumours and the immune system."
Antonio Rullan, co-lead author and clinical research fellow, Tumour Cell Biology Laboratory, Francis Crick Institute, London, United Kingdom.
Research will continue in this work to study exactly how cytoplasm is transferred from one cell to another.
Abstract
Cancer-associated fibroblasts (CAFs) perform diverse roles and can modulate therapy responses1. The inflammatory environment within tumours also influences responses to many therapies, including the efficacy of oncolytic viruses2; however, the role of CAFs in this context remains unclear. Furthermore, little is known about the cell signalling triggered by heterotypic cancer cell–fibroblast contacts and about what activates fibroblasts to express inflammatory mediators1,3. Here, we show that direct contact between cancer cells and CAFs triggers the expression of a wide range of inflammatory modulators by fibroblasts. This is initiated following transcytosis of cytoplasm from cancer cells into fibroblasts, leading to the activation of STING and IRF3-mediated expression of interferon-?1 and other cytokines. Interferon-?1 then drives interferon-stimulated transcriptional programs in both cancer cells and stromal fibroblasts and ultimately undermines the efficacy of oncolytic viruses, both in vitro and in vivo. Further, targeting IRF3 solely in stromal fibroblasts restores oncolytic herpes simplex virus function.
Authors
Esther N. Arwert, Emma L. Milford, Antonio Rullan, Stefanie Derzsi, Steven Hooper, Takuya Kato, David Mansfield, Alan Melcher, Kevin J. Harrington and Erik Sahai.
Acknowledgements
The authors thank P. Chakravarty for assistance with Bioinformatics, S. Foo and R. Sadri for technical assistance, Joan Massagué for discussion, colleagues in our laboratory for support and advice and A. Blanchard for provision of compounds. E.N.A, E.L.M., A.R., S.D., S.H. and E.S. are supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001144), the UK Medical Research Council (FC001144) and the Wellcome Trust (FC001144). E.N.A. was additionally supported by the Wellcome Trust (096084/B/11/Z). A.R. was supported by the Spanish Society for Medical Oncology (Beca Fundación SEOM), E.L.M. was supported by a Biotechnology and Biological Sciences Research Council–GlaxoSmithKline CASE Fellowship. K.J.H., A.M. and D.M. are supported by The Royal Marsden and The Institute of Cancer Research National Institute for Health Research Biomedical Research Centre and a Cancer Research UK grant (A23275). T.K. was funded by Marie-Curie action (HeteroCancerInvaison # 708651) and the Japanese Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation.
The authors declare no competing interest.
Return to top of page.
| |
|
Jun 8 2020 Fetal Timeline Maternal Timeline News
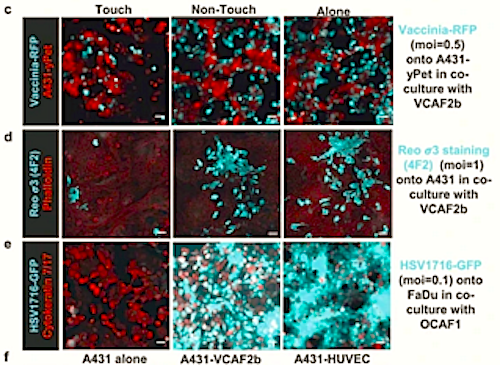 Figure 6: Cancer cell CAFs ( cancer associated fibroblasts) - resist oncolytic virus therapy [an oncolytic virus prefers to infect and kill cancer cells]. As infected cancer cells are destroyed, they release infected virus particles — called virions— that help destroy the remaining tumour. Wikipedia.
|



