|
|
Developmental Biology - Immune Response
Why Developing Nerve Cells Go Wrong
Without UBE2K enzyme, neural stem cells stop maturing...
Scientists from CECAD, Cluster of Excellence Cellular Stress Responses in Aging-Associated Diseases, have found diseases involving neurons can be explained by the loss of a certain enzyme, UBE2K.
Located in Cologne, Germany, CECAD scientists study the interactions of histones in our immortal human embryonic stem cells (hESCs). The research is published in the journal: Nature: Communications Biology.
Histones are proteins in the cell nuclei that, when massed together, become a 'spool' like structure which DNA can wind around. Winding DNA around histones, shortens DNA by a ratio of 1:10 millions, allowing long DNA strands to fit within the compact cell nucleus.
Human embryonic stem cells have a unique architecture due to their especially low levels of histone H3 and its three methylgroups (H3K9me3).
Histones regulate how genes are expressed by compressing DNA to make it fit within the cell. This compression also reflects on how genes produce proteins, the rate at which embryonic stem cells change into other cell types, and higher specialization - also known as: cell differentiation.
CECAD scientists found that embryonic stem cells exhibit high expression of UBE2K (Ubiquitin-conjugating enzyme E2 K) which is important in degrading proteins. Their loss in embryonic stem cells causes an increase in levels of H3K9 trimethyltransferase SETDB1.
Higher levels of H3K9 results in higher trimethylation of H3K9 and represses neurogenic genes during the differentiation of stem cells.
This impairs the ability of stem cells to become neural progenitor cells — precursor cells to neurons and other cells of the central nervous system.
CECAD scientists also found UBE2K tightly binds to histone H3 using the 26S proteasome, which 'kills' it (polyubiquitination).
Notably, ubc-20, the worm version of UBE2K, also regulates both histone H3 levels and H3K9 trimethylation in germ [sex] cells of the model organism: C. elegans.
"Our results indicate that UBE2K crosses evolutionary boundaries to promote histone H3 degradation and reduce H3K9me3 repressive marks in immortal cells like human ebryonic stem cells and germline cells.
As we found a link between the ubiquitin-proteasome system and epigenetic regulation in immortal human stem cells, it would be interesting to see if UBE2K regulates the epigenetic state in other cell types, like cancer cells, as well."
Azra Fatima, Post Doctural Candidate.
Different diseases like Huntington's disease are associated with alterations in epigenetic marks, and as epigenetic marks are reversible, it will be interesting to study if the epigenetic status of pluripotent stem cells from patients can be modified by controlling the proteasome system and UBE2K.
In order to correct a specific disease phenotype, novel strategies could be designed to correct epigenetic alterations in early developmental stages and thus provide potential treatment.
David Vilchez PhD, corresponding author of the manuscript, added: 'We believe that our findings can have important implications to understand the development of the human brain.'
Embryonic stem cells (ESCs) can replicate indefinitely and retain their potential to differentiate into all other types of cells. This is how nerve cells (neurons), muscle cells and all other cells of the body produce a developing organism.
Errors during this process can lead to congenital diseases. Degrading damaged proteins within the cell is an important factor in this process. Scientists study the interactions between the proteasome, the main protagonist in terminating proteins, and the epigenetic landscape — in order to understand both normal and abnormal development.
Epigenetics are heritable changes to an organism that are not determined by DNA — but through changes to chromatin, which can organize and silence DNA into tighter packages.
Abstract
Histones modulate gene expression by chromatin compaction, regulating numerous processes such as differentiation. However, the mechanisms underlying histone degradation remain elusive. Human embryonic stem cells (hESCs) have a unique chromatin architecture characterized by low levels of trimethylated histone H3 at lysine 9 (H3K9me3), a heterochromatin-associated modification. Here we assess the link between the intrinsic epigenetic landscape and ubiquitin-proteasome system of hESCs. We find that hESCs exhibit high expression of the ubiquitin-conjugating enzyme UBE2K. Loss of UBE2K upregulates the trimethyltransferase SETDB1, resulting in H3K9 trimethylation and repression of neurogenic genes during differentiation. Besides H3K9 trimethylation, UBE2K binds histone H3 to induce its polyubiquitination and degradation by the proteasome. Notably, ubc-20, the worm orthologue of UBE2K, also regulates histone H3 levels and H3K9 trimethylation in Caenorhabditis elegans germ cells. Thus, our results indicate that UBE2K crosses evolutionary boundaries to promote histone H3 degradation and reduce H3K9me3 repressive marks in immortal cells..
Authors
Azra Fatima, Dilber Irmak, Alireza Noormohammadi, Markus M. Rinschen, Aniruddha Das, Orsolya Leidecker, Christina Schindler, Víctor Sánchez-Gaya, Prerana Wagle, Wojciech Pokrzywa, Thorsten Hoppe, Alvaro Rada-Iglesias and David Vilchez.
Acknowledgements
The Deutsche Forschungsgemeinschaft (DFG) (VI742/1-2 and Germany’s Excellence Strategy-CECAD, EXC 2030-390661388), the Else Kröner-Fresenius-Stiftung (2015_A118) and the European Research Council (ERC Starting Grant-677427 StemProteostasis) supported this research. This work was also supported by ERC Consolidator Grant-616499 to T.H., the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund (POIR.04.04.00-00-5EAB/18-00) to W.P., and the Polish National Science Center (UMO-2016/23/B/NZ3/00753) to A.D. We thank L. Kurian for helpful discussions and comments on the manuscript. We thank the CECAD Proteomics Facility and the Cologne Center for Genomics (CCG) for their contribution and advice in proteomics and RNA sequencing experiments, respectively.
Return to top of page.
|
|
Jun 17 2020 Fetal Timeline Maternal Timeline News
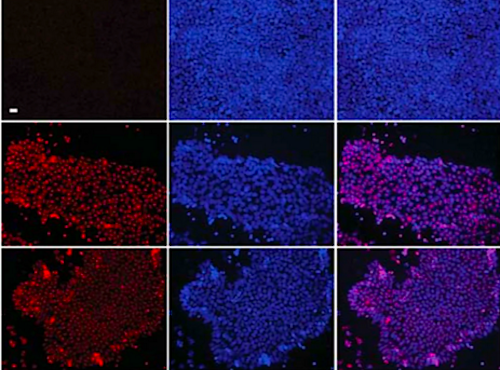 Immuno-precipitation using UBE2K and control FLAG antibodies within H9 hESCs. These images represent three independent experiments. See Figure 4 online.
|
|

