|
|
Developmental Biology - Immune Response
How Moms' Epigenetics Guarantee Her Child's Success
How an epigenetic modification drives genes to activate in a mom's offspring...
Human mothers grow babies for nine months, and after giving birth proceed to spend years raising, nurturing and teaching her children, how to both perform basic tasks and survival tasks.
Fruit flies, on the other hand, lay their eggs and then leave them to develop on their own. An irresponsible parenting decision to us humans. But to Asifa Akhtar, Max Planck Institute of Immunobiology and Epigenetics in Freiburg, Germany, it suggests something different. Fruit fly moms are simply ensuring their offspring's success by following an genetically encoded epigenomic manual deep inside their own bodies.
From Genome to Epigenome
We inherit genetic information from our parents through our DNA. However, even though all cells in our body contain the same DNA, cells "express" or 'turn on' different genes as needed — to fulfill unique functions.
DNA is wrapped around histone proteins to form a single unit called the nucleosome. Many nucleosomes joined together form chromatin which is located in the nucleus of every cell.
Epigenetic modifications happen when chemical groups are added to histones, which leads to changes in chromatin organization and can trigger either:
(1) gene activation ("expression") or
(2) gene silencing (turning gene function off).
Epigenetics represents an additional layer of information helping cells determine which genes to activate. Despite their common genome, cells in our body possess different "epigenomes".
Intergenerational Information transferred from Mom
When parents' germ cells, female oocyte and male sperm, fuse together during fertilization, they create a new organism. It was thought most epigenetic marks erase with each new fertilization. Thus, allowing all genes to be read anew with each new individual created.
Now, scientists from the laboratory of Asifa Akhtar have discovered how one particular histone modification, acetylation of histone H4 on the 16th lysine H4K16ac is added from the mother's oocyte to her young embryo.
H4K16ac is an epigenetic modification typically associated with making genes active. However, we know genes are not expressed in either the oocyte or during the first 3 hours of an embryo's life.
This begs the question: "What is H4K16ac doing at this early stage?" asks Asifa Akhtar. Investigating the function of this histone mark in early fruit fly development, the team examined numerous DNA regions "marked" by H4K16ac during early developmental stages — and before those first 3 hours when genes become activated.
Mom's Epigenetic Advice Essential to Embryonic Development
The importance of H4K16ac for the offspring became apparent when the mother failed to transmit this mark to her children. The scientists designed experiments using genetic approaches and transgenic flies to remove the enzyme MOF from fly mothers. MOF is known to be responsible for the deposition of the H4K16ac modification.
Remarkably, when the scientists studied fly offspring laid without H4K16ac information, those flies' genes weren't appropriately expressed — and their chromatin organization was severely disrupted. The majority of embryos without the maternal H4K16ac instructions subsequently died from catastrophic developmental defects.
Lessons From Flies to Humans
H4K16ac has an instructive function in the germline and is indispensable to embryonic development. It is almost like the mother leaves sticky notes with instructions on where to find the food or who to call in an emergency and so on, when a child is home alone for the first time", explains Maria Samata, the first author on the study.
"The fact that fly mothers ensure their offspring's success through epigenetics even before they are conceived is a fascinating result," explains Asifa Akhtar.
Researchers next turned to mammals to find that female mice also pass the H4K16ac histone modification to their progeny through their oocytes — raising the intriguing possibility that humans might also use mother's H4K16ac as a "blueprint" for successful embryonic development.
Whether this is the case and what information this blueprint might encode are open questions for future investigation.
Highlights
• CHRNA2 signaling in adipocytes mediates systemic energy homeostasis in vivo
• Acute high fat diet feeding activates CHRNA2 signaling in beige adipocytes
• CHRNA2 signaling regulates both UCP1- and creatine-mediated pathways
• CHRNA2 signaling regulates the activation of glycolytic beige adipocytes
Authors
Heejin Jun, Yingxu Ma, YongChen, Jianke Gong, Shanshan Liu, Jine Wang, Alexander J. Knights, Xiaona Qiao, Margo P. Emont, Shawn Xu, Shingo Kajimura and Jun Wu.
Acknowledgements
Summary
Maintaining energy homeostasis upon environmental challenges, such as cold or excess calorie intake, is essential to the fitness and survival of mammals. Drug discovery efforts targeting ?-adrenergic signaling have not been fruitful after decades of intensive research. We recently identified a new beige fat regulatory pathway mediated via the nicotinic acetylcholine receptor subunit CHRNA2. Here, we generated fat-specific Chrna2 KO mice and observed thermogenic defects in cold and metabolic dysfunction upon dietary challenges caused by adipocyte-autonomous regulation in vivo. We found that CHRNA2 signaling is activated after acute high fat diet feeding and this effect is manifested through both UCP1- and creatine-mediated mechanisms. Furthermore, our data suggested that CHRNA2 signaling may activate glycolytic beige fat, a subpopulation of beige adipocytes mediated by GABP? emerging in the absence of ?-adrenergic signaling. These findings reveal the biological significance of the CHRNA2 pathway in beige fat biogenesis and energy homeostasis.
The research was supported by the National Institutes of Health, American Diabetes Association, Chinese Scholarship Council and Michigan Life Sciences Fellows program.
Return to top of page.
|
|
Jun 22 2020 Fetal Timeline Maternal Timeline News
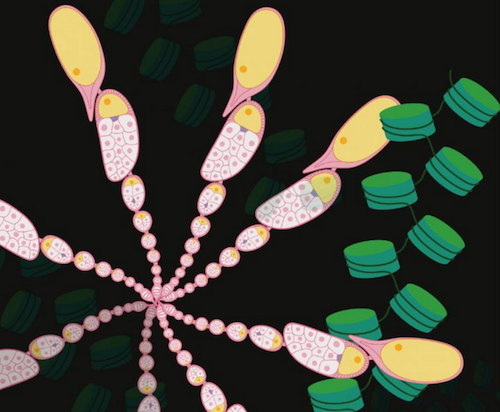 Mom's epigenetic modification of gene: H4K16ac restructures chromatin organization (GREEN) and drives gene activation in fly ovaries (PINK) CREDIT © MPI of Immunobiology and Epigenetics, M. Samata; created using Biorender.com
|
|

