|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - Autophagy Water Loss Stimulates Cell Waste Clean Out Generally it's agreed fasting (food deprivation) can kickstart this cellular degrading and recycling process. Other than that, little is known about how cells and organs control the quality of their protein molecules - or which environmental influences give the signal to start a 'clean up'. Water Loss Induces Lysosome and Autophagy Now a new trigger has been identified by scientists from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) in Berlin: Osmotic Stress. Osmotic stress kick starts the system of autophagy and lysosomal degradation. "When dehydration occurs, we suddenly see more lysosomes in cells, as more organelles degrade. It's a clever adaptation as cellular water loss simultaneously starts the aggregation (clumping) of proteins, which works better when cells have more lysosomes. Aggregates must be removed quickly to ensure continuously smooth cell function." NHE7 Switches On Newly Discovered Pathway In dehydration, the ion transporter NHE7 moves from the cell interior, it's normal position, to the cell plasma membrane which shields the cell from exterior influences. This leads to an influx of sodium ions into the cell, increasing the level of calcium in the cytosol - which acts as a key messenger. The elevated level of calcium in turn activates a transcription factor called TFEB, which finally switches on autophagy and lysosomal genes. Simply put, the system is initiated by NHE7 ion transporter, triggered by osmotic stress. "This pathway was completely unknown. It is a new mechanism that responds to a completely different type of physiological challenge to those previously known." Discovered - Aggregated Proteins in Brain Cells Counter experiments reveal the importance of this pathway for human health. When researchers removed a component of the signaling pathway - such as the transporter NHE7 or the transcription factor TFEB - aggregated protein molecules accumulated in astrocytes under osmotic stress conditions and could not be broken down. In the study, this phenomenon was demonstrated for components such as synuclein - a protein that plays a role in Parkinson's disease. "Neurodegenerative diseases in particular are a possible consequence of this pathway being switched on incorrectly. In addition, NHE7 is a so-called Alzheimer's risk gene. We now have new insights into why this gene could play such a critical role." Another point of interest is an intellectual disability in boys passed via the X chromosome. This disability is due to a mutation in the NHE7 gene. Researchers suspect the disease mechanism is linked to the degradation mechanism now described. If only the switch, i.e. the NHE7 protein, were defective, an attempt could be made to turn on the pathway in another way. "It is very difficult in practice, and extremely expensive, to repair a genetic defect, but it would be conceivable to pharmacologically influence the NHE7 protein or to use other stimuli such as spermidine as a food supplement to switch on the autophagy system in these patients," explains cell biologist and neurocure researcher Volker Haucke. Medical Relevance of Basic Research In order to carry out such interventions, however, the foundations need to be researched more thoroughly. For example, it is not yet clear how osmotic stress affects the translocation of NHE7 to the cell surface. It is also not known whether the entire degradation system is initiated or whether just individual genes are switched on, or which specific responses to osmotic stress are needed to activate the lysosomal system. Nor is it known which other stimuli may be triggered by this physiological process. Researchers now seek to answer all these questions in subsequent projects. This study is published in the prestigious journal Nature Cell Biology. Describing the new signaling pathway in detail, provides a basis for improving our understanding of environmental influences on our cell recycling and degradation system. Now, this knowledge can be used for therapeutic purposes. "Our work shows the fundamental impact water and ionic balance have on cells and tissue in breaking down defective protein molecules. Now we want a better understanding of this mechanism as it plays a major role in aging, neuron degeneration and prevention of other diseases." Abstract Lysosomes serve as cellular degradation and signalling centres that coordinate metabolism in response to intracellular cues and extracellular signals. Lysosomal capacity is adapted to cellular needs by transcription factors, such as TFEB and TFE3, which activate the expression of lysosomal and autophagy genes. Nuclear translocation and activation of TFEB are induced by a variety of conditions such as starvation, lysosome stress and lysosomal storage disorders. How these various cues are integrated remains incompletely understood. Here, we describe a pathway initiated at the plasma membrane that controls lysosome biogenesis via the endocytic regulation of intracellular ion homeostasis. This pathway is based on the exo-endocytosis of NHE7, a Na+/H+ exchanger mutated in X-linked intellectual disability, and serves to control intracellular ion homeostasis and thereby Ca2+/calcineurin-mediated activation of TFEB and downstream lysosome biogenesis in response to osmotic stress to promote the turnover of toxic proteins and cell survival. Authors Tania López-Hernández, Dmytro Puchkov, Eberhard Krause, Tanja Maritzen and Volker Haucke. Acknowledgements The authors thank S. Hahn, D. Löwe, S. Zillmann and C. Schmidt for technical assistance, M. Poet, D. Owen, D. Sabatini, S. Ferguson and D. Rubinsztein for constructs, and T. Jentsch for discussion. This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC-2049–390688087) and grants to V.H. (HA2686/13-1; Reinhart-Koselleck-Award). Return to top of page.
|
| Jul 10 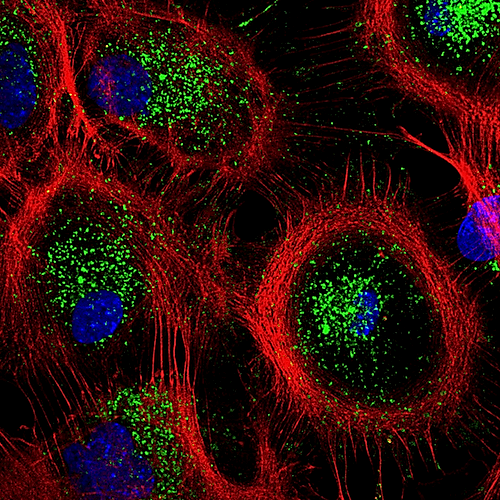 Mouse astrocytes showing their actin cytoskeleton (RED) and lysosomes (GREEN) CREDIT Tania Lopez-Hernandez
| |||||||||||||||||||||||||||

