|
|
Developmental Biology - Brain Diseases
Human 'Biofilm' Causing Alzheimer/Arthritis Diseases?
Salmonella biofilm protein causes autoimmune responses with a possible link to Alzheimer's...
A Salmonella biofilm protein can cause autoimmune responses that we have interpreted an Alzheimer's brain disorder and even as arthritis.
University of Saskatchewan scientists from the Vaccine and Infectious Disease Organization (USask), International Vaccine Centre (VIDO-InterVac) and Temple University (Philadelphia, Pennsylvania, USA) have demonstrated that a Salmonella biofilm protein can cause autoimmune responses and arthritis in animals.
Previously salmonella was thought to only form biofilms in the environment, such as on food processing surfaces.
Biofilms are dense collections of bacteria that stick together on surfaces to protect that bacteria from harsh conditions, including antibiotics and disinfectants. Detecting biofilms in an animal during an infection was a surprise.
In research published in PLOS Pathogens, a VIDO-InterVac team led by Aaron White discovered that Salmonella biofilms form in the intestines of infected mice. For the study, the team used a mouse model to replicate human food-borne illness and show that a biofilm protein called "curli" grows on surface of bacteria and is connected to "negative" health outcomes.
Curli are a special type of protein called amyloids. Similar human proteins have been associated with neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and Amyotrophic lateral sclerosis (ALS or Lou Gehrig's disease).
Scientists don't know how these diseases start, but have speculate that something must "trigger" these accumulations of amyloids.
"We are the first to show a food-borne pathogen can make these types of proteins in the gut.
There has been speculation bacteria can stimulate amyloid plaque formation in Alzheimer's, Parkinson's and ALS contributing to disease progression.
Discovery of curli in the gut could represent an important link, pointing to a potentially infectious cause for these diseases."
Aaron P. White PhD, Vaccine and Infectious Disease, International Vaccine Centre (VIDO-InterVac), University of Saskatchewan (USask), Canada; Temple University, Philadelphia, Pennsylvania, USA; a leading expert on salmonella biofilms and curli amyloids, and a study author.
"In mice, these reactions were triggered within six weeks of infection, demonstrating that curli can be a major driver of autoimmune responses."
Çagla Tükel PhD, Associate Professor of Microbiology & Immunology, Temple University; led her team to determine the presence of curli leads to autoimmunity and arthritis - both known to be complications of Salmonella infections in humans; also a study author.
The next step in the research is to confirm that this also occurs in humans, and test if other food-borne pathogens related to Salmonella can cause similar autoimmune reactions.
"This important discovery suggests food-borne pathogens could initiate or worsen autoimmunity and have the potential to contribute to amyloid disorders such as Alzheimer's and Parkinson's disease."
Dr. Volker Gerdts, Director, Vaccine and Infectious Disease Organization - International Vaccine Centre (VIDO-InterVac) University of Saskatchewan, Canada.
Abstract
Highlights
Reactive arthritis, an autoimmune disorder, occurs following gastrointestinal infection with invasive enteric pathogens, such as Salmonella enterica. Curli, an extracellular, bacterial amyloid with cross beta-sheet structure can trigger inflammatory responses by stimulating pattern recognition receptors. Here we show that S. Typhimurium produces curli amyloids in the cecum and colon of mice after natural oral infection, in both acute and chronic infection models. Production of curli was associated with an increase in anti-dsDNA autoantibodies and joint inflammation in infected mice. The negative impacts on the host appeared to be dependent on invasive systemic exposure of curli to immune cells. We hypothesize that in vivo synthesis of curli contributes to known complications of enteric infections and suggest that cross-seeding interactions can occur between pathogen-produced amyloids and amyloidogenic proteins of the host.
Author summary
Our manuscript focuses on curli, a ‘functional amyloid’ produced by Salmonella as well as other enteric bacteria. We present the first biochemical evidence that these fibers are produced in the gastrointestinal tract of mice after oral infection, the natural route for Salmonella infections. This finding is significant because of the immune impacts on the host; we show that curli cause an increase in autoimmunity and inflammation in the knee joints of infected mice. Reactive arthritis is a known autoimmune complication after enteric infections and our results indicate that presence of curli in the gut provides a novel linchpin of pathogenesis. As curli or curli-like amyloids are also produced by the commensal bacteria, it is possible that the unintended release of amyloids produced by the microbiota could trigger similar autoimmune reactions. Finally, our work provides conceptual evidence for the possibility of cross-seeding between bacterial amyloids like curli and human amyloids involved in amyloid-associated diseases such as Alzheimer’s Disease via the gut microbiome or infections.
Authors
Amanda L. Miller, J. Alex Pasternak, Nicole J. Medeiros, Lauren K. Nicastro, Sarah A. Tursi, Elizabeth G. Hansen, Ryan Krochak, Akosiererem S. Sokaribo, Keith D. MacKenzie, Melissa B. Palmer, Dakoda J. Herman, Nikole L. Watson, Yi Zhang, Heather L. Wilson, R. Paul Wilson, Aaron P. White and Çagla Tükel.
Acknowledgements
The work was supported by Nina Ireland and the National Institutes of Health (R01NS099099, R01MH109907, U01MH116438, U01MH105989).
The Canadian research was supported by funding from the Natural Sciences and Engineering Research Council of Canada and from Stephen Jarislowsky to Aaron White who holds The Jarislowsky Chair in Biotechnology at USask.
The authors would like to thank VIDO animal care staff for help with planning and performing the animal experiments. We would like to thank Andreas Baumler, Scott Napper, Nick Feasey, and Phil Cohen for critical reading of manuscript and Bill Kay, Marc Monestier, Roberto Caricchio, and Stefania Gallucci for helpful discussions.
Return to top of page.
|
|
Jul 15 2020 Fetal Timeline Maternal Timeline News
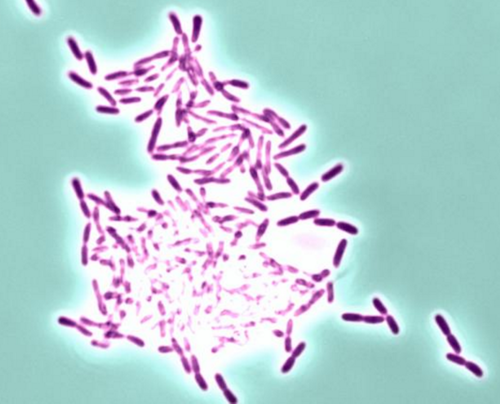 This is Pseudomonas bacteria forming a biofilm. CREDIT Vernita Gordon, University of Texas at Austin.
|
|

