|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - Nucleolus Nucleolus Controlls Cell Growth "To get the characteristic layered, 'core-shell' architecture of the nucleolus, all you need to do is mix the right molecules together at sufficient concentrations." Brangwynne and colleagues had previously shown how a number of other organelles besides the nucleolus are also phase-separated liquid droplets of RNA and protein. The present findings point to how these organelles — all of which lack compartmentalization offered by membranes — exhibit intricate architectures tailored to their cellular duties. "The basic properties of fluids dictate which components of the nucleolus are on the inside and which are on the outside. As the fundamental principles underlying this effect are observed even in non-living matter, we think this physical picture applies to many organelles inside cells." Researchers investigated nucleoli by conducting experiments using purified nucleolar proteins from frog eggs, roundworms, cultured cells, along with computer modeling. Spearheaded by Feric, the work on frog eggs took advantage of their large size and multiple, large nucleoli. Frog eggs nucleoli normally do not come into contact with each other due to an elastic actin network. Feric incubated the eggs in a pharmacological drug breaking down the actin, allowing nucleoli to contact one another. He then observed nucleoli fuse, much like two droplets of water. By analyzing these fusion events, researchers teased out the differing biophysical properties of their nucleolar layers. Meanwhile, Vaidya led the protein-based lab work, to find droplets of the two main nucleolar proteins, dubbed FIB1 and NPM1, could not blend into each other as a homogenous fluid. FIB1's surface tension was higher than NPM1, precisely mimicking the nucleolar structure in living cells. As a demonstration of this, researchers also created multi-phase drops - liquids embedded within other liquids - using vegetable oil and silicone oil in water. Altogether, the data easily explains how individual subcompartments of the nucleolus nestle inside of each other, somewhat like a Russian "nesting" doll. Layering of Form - Follows Function Newly made RNA molecules proceed from the organelle's core into its middle. Outer components, receiving modifications as they move - as if on an assembly line in a factory - churn out RNA bits, which upon leaving the nucleolus enter into the cell's cytoplasm and link up to form ribosomes. Factories in their own right, ribosomes manufacture a cell's many thousands of proteins. Beyond making ribosomes, the nucleolus has lately emerged as a hub for coordinating cellular growth, helping to regulate cell division and even setting the timing of a cell's self-destruction in reaction to stress or damage. Given this centrality, the nucleolus is also increasingly being recognized for roles in disease. For instance, during certain illnesses, nucleoli may lose some of their normal liquidness. If the faulty nucleoli become more fibrous, throwing off kilter the smooth flow of RNA through their chambers, this could contribute to heart and neurological diseases. Another major disease nucleoli figure prominently in is cancer. In malignant cells, hijacked nucleoli overproduce proteins to fuel out-of-control, rapid divisions. Oncologists routinely grade cancer cells' aggressiveness in part on the degree of their misshapen and bloated nucleoli. Further insight into the normal layout of nucleoli, as the new Cell study provides, will thus help in assessing the organelle as a promising therapeutic target for drugs in near future. Brangwynne also hopes that understanding nucleolar core-shell structure will inform his lab's ongoing studies into other overlooked organelles without membranes, like stress granules, p-bodies and Cajal bodies. Although these structures barely turn up in biology text books, recent work has suggested they also play key roles in both cell physiology and many diseases, including aggregation diseases such as Alzheimer's and amyotrophic lateral sclerosis (ALS). "It's amazing how complex structures like the nucleolus can self-organize, based on relatively simple physical principles. We're hoping that there is also simplicity underlying how such organization can go awry in disease." Abstract Liquid-liquid phase separation has emerged as a key process underlying intracellular organization, including the regulation of RNA/protein assemblies, and their dysregulation in disease. However, the molecular mechanisms that drive these phase transitions, the biophysical properties of the resulting droplets, and the way their properties impact biological function, remain poorly understood. Here, we focus on LAF-1, an essential DEAD-box RNA helicase associated with P granules in the C. elegans germline. We find that purified LAF-1 can phase separate into liquid droplets at near physiological (µM) concentrations. LAF-1 droplet formation is driven by its disordered N-terminal RGG domain, which is both necessary and sufficient for droplet formation. We combine microrheology, FRAP, and single molecule imaging approaches to reveal the local viscoelastic properties and molecular dynamics inside the droplets. Our results provide mechanistic and structural insight into the phase transition-driven assembly of liquid-like organelles, and suggest that the biophysics of intracellular phase separation can sensitively control molecular dynamics and function. Authors Shana Elbaum, Younghoon Kim, Sua Myong and Clifford Brangwynne. Other Princeton co-authors on the study are graduate student Lian Zhu and junior Tiffany Richardson, both in Brangwynne's Soft Living Matter Group. Other authors include: Tyler Harmon and Rohit Pappu of Washington University in St. Louis; Diana Mitrea and Richard Kriwacki of St. Jude's Research Hospital, Memphis. Funding for the research was provided in part by the National Science Foundation, the National Institutes of Health, the National Cancer Institute and the Helen Hay Whitney Foundation. Return to top of page.
|
| Jul 23 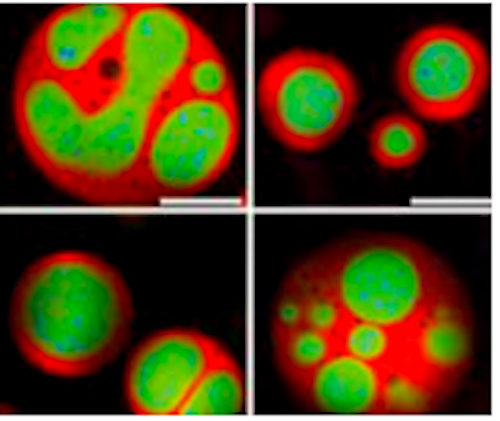 Although known since the 1830s as a round, dark spot in a cell's nucleus, only recently has the nucleolus gotten its full due. Scientists have learned that besides building a cell's protein factories, this specialized subunit, or organelle, serves more broadly as a control center for cellular growth and health. CREDIT Princeton University.
| |||||||||||||||||||||||||||

