|
|
Developmental Biology - Optogenetics
Fly Embryo Can Be Controlled By Simple Light Signals
Multiple early organ systems respond to light activated Ras/Erk protein pathway...
Princeton researchers have discovered that embryo development in the fruit fly (Drosophila Melanogaster) is governed by proteins contributed by its mother while the embryo is still a simple and undefined cell.
A mother drosophila deposits more of her own cells around that embryo cell, contributing proteins into very specific locations that stimulate the formation of 2 axis: (1) the anterior-posterior (A — P) and (2) ventral-dorsal (V — D). Slightly later, she will add another round of proteins - which researchers call terminal patterning - to guide development of the Head to Tail axis.
Terminal patterning is driven by a protein called Torso which deposited in the embryo, only binds to proteins located at the embryo's anterior and posterior ends.
Torso stimulates several signal cascades. One called Ras/ERK, which activates crucial genes. Without Torso or the proteins which it binds to, embryos fail to develop head and tail structures and eventually die.
"At this point, most developmental patterns have been studied in great detail, so biologists know when and where they appear and disappear. What is unknown is exactly what information is contained in a pattern?"
Jared E. Toettcher PhD, Department of Molecular Biology, Princeton University, Princeton, New Jersey, USA.
Traditional approaches to studying functions of a given protein can't manipulate that function with enough precision to answer such a question. However, Toettcher's lab used the emerging technology of "optogenetics" to develop a new research tool: a protein called OptoSOS that allows researchers to activate the Torso signaling pathway while OptoSOS is illuminated with blue light.
"This is great because we can produce light patterns with high precision, allowing us to draw any pattern that we would like onto the embryo."
Jared E. Toettcher PhD.
To investigate where and when Ras/ERK signaling is needed for terminal patterning, Johnson and his colleagues expressed OptoSOS in embryos of flies without Torso signaling. They then placed the embryos under a microscope and precisely stimulated their anterior and posterior ends with light long enough to mimic the natural duration of Ras/ERK signaling.
Remarkably, all embryos left in the dark died — failing to develop properly — while about one third of light-stimulated embryos developed normally. Despite the trauma of extraction and growing in a microscope chamber, these embryos formed head and tail structures, eventually hatched and reached adulthood. Female flies created this way even laid eggs — although, as expected, the eggs were sterile because, like their mothers, they didn't have Torso signaling. But, unlike their mothers, their eggs were unstimulated by light.
Much of terminal patterning is driven by two genes whose expression is controlled by the Ras/ERK pathway in response to Torso signaling. In normal embryos, expression of these two genes occurs in different cells at different times. In light-stimulated OptoSOS embryos without Torso signaling, expression of the two genes overlapped in both space and time, suggesting precise expression patterns are not required for development.
The authors next investigated whether different aspects of the developmental program are triggered at the same or different stimulus thresholds. To do this, they monitored embryonic development after varying the intensity and duration of light stimulus in OptoSOS embryos lacking Torso signaling.
Together, this data — published in Current Biology on July 23, 2020 — suggests that what appears to be a very complex developmental program is actually under the control of a relatively simple system depending on different thresholds of Ras/Erk signaling.
"These findings demonstrate the power of using optogenetics to activate and inactivate signaling pathways in time and space. Even more amazingly, they are able to tune the strength of the signal and obtain precise quantitative information about the mechanism in the living embryo."
Gertrude Schüpbach PhD, instrumental in identifying components of terminal patterning.
"We found that the terminal pattern appears to work as a series of switches, where successively longer light pulses trigger a predictable sequence of body parts being 'rescued' one by one. We think this is the beginning of mapping when and where to produce specific body structures."
Jared E. Toettcher PhD.
Abstract
Animal embryos are patterned by a handful of highly conserved inductive signals. Yet, in most cases, it is unknown which pattern features (i.e., spatial gradients or temporal dynamics) are required to support normal development. An ideal experiment to address this question would be to "paint" arbitrary synthetic signaling patterns on "blank canvas" embryos to dissect their requirements. Here, we demonstrate exactly this capability by combining optogenetic control of Ras/extracellular signal-related kinase (ERK) signaling with the genetic loss of the receptor tyrosine-kinase-driven terminal signaling patterning in early Drosophila embryos. Blue-light illumination at the embryonic termini for 90 min was sufficient to rescue normal development, generating viable larvae and fertile adults from an otherwise lethal terminal signaling mutant. Optogenetic rescue was possible even using a simple, all-or-none light input that reduced the gradient of Erk activity and eliminated spatiotemporal differences in terminal gap gene expression. Systematically varying illumination parameters further revealed that at least three distinct developmental programs are triggered at different signaling thresholds and that the morphogenetic movements of gastrulation are robust to a 3-fold variation in the posterior pattern width. These results open the door to controlling tissue organization with simple optical stimuli, providing new tools to probe natural developmental processes, create synthetic tissues with defined organization, or directly correct the patterning errors that underlie developmental defects.
Authors
Heath E. Johnson, Nareg J.V. Djabrayan, Stanislav Y. Shvartsman and Jared E. Toettcher.
Acknowledgements
The authors thank Trudi Schu¨ pbach and Yogesh Goyal for helpful discussions during the planning stages of our work, Romain Levayer for sharing the miniCic biosensor, Gerardo Jimenez for sharing DNA constructs used to design transcriptional reporters of tll and hkb, and Shannon Keenan for testing and characterization of the MS2 reporters lines. H.E.J. was supported by the NIH Ruth Kirschstein fellowship F32GM119297. This work was also supported by NIH grant DP2EB024247 and NSF CAREER Award 1750663 (to J.E.T.) and NIH grant 5R01HD085870 (to S.Y.S.). We also thank Dr. Gary Laevsky and the Molecular Biology Microscopy Core, which is a Nikon Center of Excellence, for microscopy support. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Return to top of page.
|
|
Jul 24 2020 Fetal Timeline Maternal Timeline News
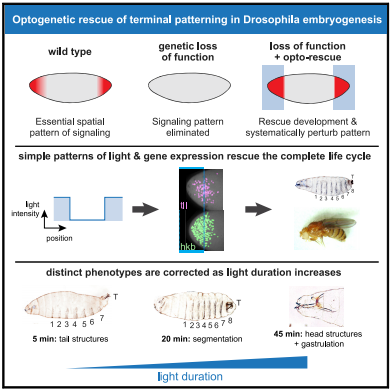 The loss of genes that determine patterns in development in a fly embryo can be rescued by light! Stimulating the Ras protein can control development and be used to probe cell and tissue regulation, engineer its organization, even correct certain defects. CREDIT The Authors.
|
|

