|
|
Developmental Biology - Wound Healing
New Insights Into How Wounds Heal
Using 3D mapping, research uncovers how wounds heal...
Our cells quickly mobilize when repairing any wounds to our skin. While we know how cells heal wounds and how scars form, research from Washington University in St. Louis, Missouri for the first time has determined how the process begins.
This new knowledge will hopefully shed light not only on how wounds heal, but on tissue remodelling that leads to scarring, and even cancer spread (metastasis).
The team discovered how fibroblasts, the common cells in connective tissue, interact with the extracellular matrix (ECM). The ECM is a 3-dimensional network of extracellular macromolecules, such as collagen, enzymes, and glycoproteins, that provide structure and biochemical support to surrounding cells. The ECM also sends out mechanical cues reflecting and affecting cell behavior.
Led by Delaram Shakiba, postdoctoral fellow at the NSF Science and Technology Center for Engineering Mechanobiology (CEMB) and McKelvey School of Engineering, the team uncovered a repeating process between skin cells and their environment — that affects structures in cells yet was previously unknown.
Results of their research published in ACS Nano on July 28.
"Clinical efforts to prevent progression of fibrocontractile diseases, such as scarring and fibrosis, have largely been unsuccessful. In part, this is because the mechanisms cells use to interact with protein fibers around them were unclear.
We found fibroblasts use completely different mechanisms in the early stages - and I think the most treatable stages of these interactions. Their responses to drugs can therefore be the opposite of what they would be in later stages."
Delaram Shakiba PhD, NSF Science and Technology Center for Engineering Mechanobiology and Department of Mechanical Engineering and Materials Science, Washington University St. Louis, St. Louis, Missouri, USA.
Genin, co-director of the CEMB, explains that the wound healing process has stymied mechanobiology research for some time. Now that they understand the process, they hope to become able to control the shape a cell takes.
Researchers have learned they can control cell shape in two ways:
• First, by controlling boundaries around a cell
• Second, by inhibiting/upregulating specific proteins that remodel collagen.
Fibroblasts pull edges of a wound together. Cellular collagen remodels the extracellular matrix, fully closing the wound. This is where mechanobiology comes into play.
"There's a balance between tension and compression inside a cell newly exposed to fibrous proteins. The tension from actin cables, plays with that balance. We can make these actin protrusions grow extremely long. We can stop cell remodeling from occurring. Or we can increase it."
Guy M. Genin PhD, NSF Science and Technology Center for Engineering Mechanobiology and Department of Mechanical Engineering and Materials Science, Washington University St. Louis, St. Louis, Missouri, USA.
The team used a 3D-mapping technique - the first time being applied to collagen - along with a computational model to calculate the 3D fields created by strain and stress inprotrusions from cells.
As cells accumulated collagen, tension-driven remodeling and alignment of collagen fibers led to the formation of collagen tracts. Collagen fiber alignment requires cooperative interaction between cells, allowing cells to interact mechanically.
"New methods of microscopy, tissue engineering and biomechanical modeling greatly enhance our understanding of the mechanisms by which cells modify and repair the tissues they populate.
Fibrous cellular structures generate and guide forces that compress and reorient their extracellular fibrous environment.
This raises new questions about the molecular mechanisms of these functions and how cells regulate the forces they exert and how they govern the extent of matrix deformation."
Elliot L. Elson PhD, Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, Missouri, USA.
"Wound healing is a great example of how these processes are important in a physiologic way," Genin adds. "We'll be able to come up with insight in how to train cells not to excessively compact the collagen around them."
Abstract
Fibroblasts undergo a critical transformation from an initially inactive state to a morphologically different and contractile state after several hours of being embedded within a physiologically relevant three-dimensional (3D) fibrous collagen-based extracellular matrix (ECM). However, little is known about the critical mechanisms by which fibroblasts adapt themselves and their microenvironment in the earliest stage of cell–matrix interaction. Here, we identified the mechanisms by which fibroblasts interact with their 3D collagen fibrous matrices in the early stages of cell–matrix interaction and showed that fibroblasts use energetically efficient hierarchical micro/nano-scaled protrusions in these stages as the primary means for the transformation and adaptation. We found that actomyosin contractility in these protrusions in the early stages of cell–matrix interaction restricts the growth of microtubules by applying compressive forces on them. Our results show that actomyosin contractility and microtubules work in concert in the early stages of cell–matrix interaction to adapt fibroblasts and their microenvironment to one another. These early stage interactions result in responses to disruption of the microtubule network and/or actomyosin contractility that are opposite to well-known responses to late-stage disruption and reveal insight into the ways that cells adapt themselves and their ECM recursively.
Authors
Delaram Shakiba, Farid Alisafaei, Alireza Savadipour, Roger A. Rowe, Zhangao Liu, Kenneth M. Pryse, Vivek B. Shenoy, Elliot L. Elson, and Guy M. Genin.
Supporting Information is available free of charge here:
Microsoft AVI: nn9b09941_si_001.avi (9.67 MB)
pdf:https://pubs.acs.org/doi/10.1021/acsnano.9b09941.
Movie S1: formation of cell protrusions (AVI)
Additional data include the theoretical matrix model (Section 1), the theoretical cytoskeletal model (Section 2), discrete fiber network simulations (Figure S1), nocodazole pretreated fibroblasts (Figure S2), cytochalasin D pretreated fibroblasts (Figure S3), Y-27632 pretreated fibroblasts (Figure S4), blebbistatin pretreated fibroblasts (Figure S5), different components of the theoretical cytoskeletal model (Figure S6), one-dimensional representation of the theoretical cytoskeletal model (Figure S7), formation of actin-based filopodium-like structures on cell protrusions (Figure S8)(PDF)
Return to top of page.
|
|
Aug 3 2020 Fetal Timeline Maternal Timeline News
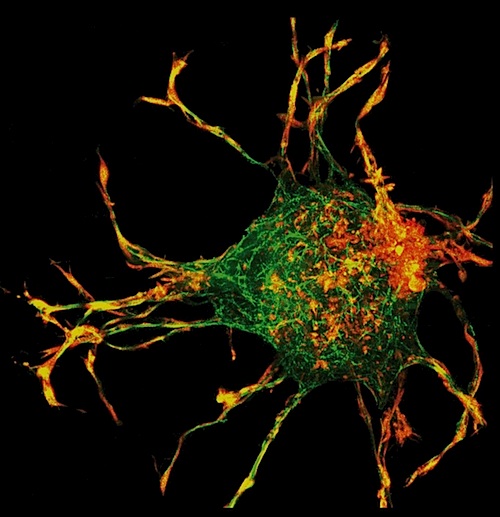 Researchers used a 3D-mapping technique - the first time applied to collagen - along with a computation calculating 3D strain and stress fields created by protrusions from the cell. CREDIT Brandie Jefferson
|
|



