|
|
Developmental Biology - Mitochondria
How to Become a Nerve Cell
Mitochondria regulate a key event in brain development — turning precursor neural stem cells into nerve cells...
Mitochondria are small organelles inside almost every body cell, particularlly in fat cells where energy is stored, and in muscle cells where energy is spent. They provide us with a continual source of energy. Energy particularly critical to our high fuel-consuming brain.
In this week's edition of Science magazine, Belgian researchers led by Pierre Vanderhaeghen (VIB, The Flanders Institute for Biotechnology in Brussels, Belgium) announce that they also found mitochondria regulate a key event in brain development — the conversion of neural stem cells into nerve cells.
Mitochondria influence neural stem cell fate change during a period of development twice as long in humans as in mice. This is an unexpected function of mitochondria. It may help explain how humans developed a bigger brain during evolution, and how mitochondrial defects lead to neurodevelopmental diseases.
Our brains are made up of billions of incredibly diverse neurons. They first arise in the developing brain when stem cells stop self-renewing and differentiate into a particular type of neuron. Called neurogenesis, this process is precisely regulated to give rise to the enormous complex structure that is our brain. It is thought small differences in the way neural stem cells generate neurons is at the origin of a dramatic increase in size and complexity of our brain.
To gain insight in this complex process, professor Pierre Vanderhaeghen, and his colleagues examined mitochondria, small organelles providing energy in every cell in our body, including our developing brain.
"Diseases caused by defects in mitochondria lead to developmental problems in many organs, in particular the brain. We used to think this was related to the crucial function of mitochondria providing energy to the cells — but this is only part of the story. Recent work in stem cells suggests mitochondria have a direct influence on organ development. We have tested whether and how this could be the case in the brain."
Pierre Vanderhaeghen PhD, a specialist in stem cell and developmental neurobiology, in the VIB-KU Leuven Center for Brain & Disease Research
Fission and Fusion
Together with his team, Vanderhaeghen explored whether and how mitochondrial remodeling is coupled with neuronal fate commitment during neurogenesis. "Mitochondria are highly dynamic organelles, that can join together (fusion) or split up (fission), and we know these dynamics are associated with fate changes in various types of stem cells," explains Vanderhaeghen.
"We found shortly after stem cells divide, daughter cell mitochondria - destined to self-renew — fuse. But, in daughter cells that become neurons — mitochondria show high levels of fission."
Ryohei Iwata, postdoctoral researcher, Vanderhaeghen lab.
This was not a coincidence, as researchers found increased mitochondrial fission promotes differentiation into neurons, while mitochondrial fusion after mitosis directs daughter cells to self-renew.
Time Window
Mitochondrial dynamics are important to become a neuron - but there is more.
"We found that the influence of mitochondrial dynamics on cell fate choice is limited to a very specific time window, right after cell division. Interestingly, the restricted time window is twice as long in humans compared to mice."
Previous findings were primarily focused on fate decisions of neural stem cells before they divide. But, our data reveals that cell fate can be influenced over a much longer period, even after neural stem cell division. This may have implications in the emerging field of cell reprogramming, where scientists try to convert non-neuronal cells into neuronal cells for therapeutic purposes.
This period of plasticity is much longer in human cells compared to mouse cells. So, it is tempting to speculate that it contributes to the increased self-renewal capacity of human progenitor cells — and thus, to our uniquely developed brain and cognitive abilities. It is fascinating that mitochondria, small organelles that evolved in cells more than a billion years ago, might have contributed to the evolution of the human brain."
Pierre Casimir, PhD student in the Vanderhaeghen laboratory.
Abstract
The conversion of neural stem cells into neurons is associated with the remodeling of organelles, but whether and how this is causally linked to fate change is poorly understood. We examined and manipulated mitochondrial dynamics during mouse and human cortical neurogenesis. We reveal that shortly after cortical stem cells have divided, daughter cells destined to self-renew undergo mitochondrial fusion, whereas those that retain high levels of mitochondria fission become neurons. Increased mitochondria fission promotes neuronal fate, whereas induction of mitochondria fusion after mitosis redirects daughter cells toward self-renewal. This occurs during a restricted time window that is doubled in human cells, in line with their increased self-renewal capacity. Our data reveal a postmitotic period of fate plasticity in which mitochondrial dynamics are linked with cell fate.
Authors
Ryohei Iwata, Pierre Casimir and Pierre Vanderhaeghen.
Acknowledgements
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works https://www.sciencemag.org/about/science-licenses-journal-article-reuse
Return to top of page.
|
|
Aug 14 2020 Fetal Timeline Maternal Timeline News
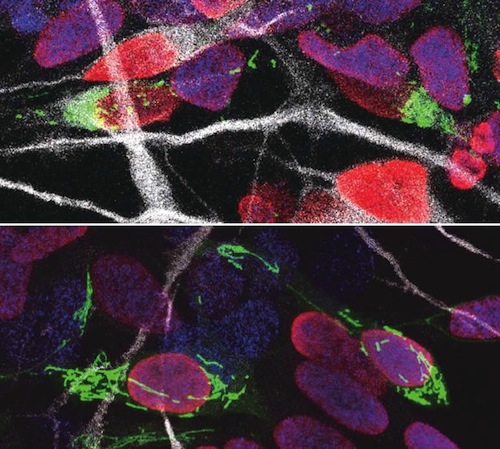 Human progenitor cells nucleus with DNA colored RED after cell division - their mitochondria labeled GREEN. (top) Human cells with fragmented mitochondria become neurons, whereas (bottom) tubular mitochondria remain progenitor cells and their DNA-containing nucleus is marked BLUE — while new born neurons are marked WHITE. CREDIT Ryohei Iwata.
|
|



