|
|
Developmental Biology - Mitochondrial Therapy
Preventing Heart Disease Before Birth
Mitochondrial therapy in complicated pregnancies lowers the chance of cardiovascular problems in baby and future adult...
Pregnancy complications can reduce oxygen in the womb, often causing low oxygen levels to the baby. Babies experiencing these conditions often develop heart disease as adults. However, a new study using sheep, finds how a specialised antioxidant, MitoQ, can prevent heart disease in its beginnings. These results are published in the journal Science Advances.
Genetics, and their interaction with lifestyle risk factors such as smoking and obesity, play a role in determining heart disease risk in adults. But there is also strong evidence that the environment experienced during sensitive periods of fetal development directly influences long-term cardiovascular health - a process known as 'developmental programming.'
Low oxygen in the womb - known as chronic fetal hypoxia - is one of the most common complications in human pregnancy. In a process termed 'oxidative stress,' low oxygen to the developing fetus can cause damage to its heart and blood vessels. Fetal hypoxia can be diagnosed when a scan during pregnancy shows the baby is not growing properly.
"Many people may be predisposed to heart disease as adults because of the low level of oxygen they received in the womb. By providing a specific mitochondria-targeted antioxidant supplement to mothers whose pregnancy is complicated by fetal hypoxia, we can potentially prevent this."
Dino A. Giussani MA, PhD, ScD, FRCOG; Professor, University of Cambridge, Department of Physiology, Development and Neuroscience.
Chronic hypoxia is common to many complications of pregnancy. It can be caused by a number of conditions including pre-eclampsia, infection of the placenta, gestational diabetes or maternal obesity.
Oxidative stress largely originates in cell mitochondria - the so called batteries that power our cells. Mitochondria are where respiration and energy are producedr. To target mitochondria, the Cambridge team used MitoQ, developed by Professor Mike Murphy and his colleagues at the University of Cambridge's MRC-Mitochondrial Biology Unit.
MitoQ selectively accumulates within mitochondria, where it works to reduce oxidative stress.
Having established the safety of the treatment, the researchers gave MitoQ to pregnant sheep under low oxygen conditions. They found that the mitochondrial therapy protects against fetal growth restriction and high blood pressure in the offspring as adults. Using chicken embryos they also showed that MitoQ protects against mitochondria-derived oxidative stress.
MitoQ has already been used in a number of human trials and seen to lower hypertension in older adults.
It is very exciting to see the potential to use MitoQ to treat a baby during a problematic pregnancy and prevent problems arising far later in life. There's still a long way to go before this can be used by pregnant mothers, but our work points to new possibilities for novel treatments," said Professor Murphy, who was also involved in the study.
This is the first time that MitoQ has been tested during sheep pregnancy. Sheep are animals whose cardiovascular development resembles that of a human baby more closely than laboratory rats and mice. Chicken embryos were also used to isolate the direct effects of MitoQ therapy on the embryonic heart independent of any influence on the mother or placenta.
"Our cardiovascular health is influenced by the lifestyle choices we make in adult life, but can also be traced back to the conditions we experienced when developing inside the womb.
This study reveals a plausible way to reduce the future risk of high blood pressure and consequent heart disease in babies from complicated pregnancies. Further research is now needed to translate these findings from animals to humans and identify the most effective time in development to give the MitoQ supplement to 'at risk' babies - whether that's a particular point during pregnancy or soon after birth. Overcoming this next hurdle will enable it to be tested in clinical trials."
James Leiper PhD, Associate Medical Director, British Heart Foundation; research: University of Glasgow, Institute of Cardiovascular and Medical Sciences.
Cardiovascular disease is a group of disorders of the heart and blood vessels that can cause heart attacks and strokes. It claims the life of one in three people, and costs the United States and Canada $130 billion US dollars; in the United Kingdom over £30 billion every year.
The majority of these costs are for treatments that improve outcomes, but do not cure the disease.
There are increasing calls within the public health community to change the focus of cardiovascular disease research from treatment to prevention. By looking at the specific circumstances that increase the risk of developing heart disease, interventions can be made as early as possible rather than waiting until disease has become irreversible.
"If we want to reduce the prevalence of cardiovascular disease, we need to think of prevention rather than a cure. Applying this concept to pregnancy complications, we can bring preventative medicine all the way back into the womb - it's treatment before birth. It completely changes our way of thinking about heart disease."
Dino A. Giussani PhD.
Abstract
The prenatal origins of heart disease in offspring have been established. However, research in species with developmental milestones comparable to humans is lacking, preventing translation of this knowledge to clinical contexts. Using sheep and chickens, two species with similar cardiovascular developmental milestones to humans, we combined in vivo experiments with in vitro studies at organ, cellular, mitochondrial, and molecular levels. We tested mitochondria-targeted antioxidant intervention with MitoQ against cardiovascular dysfunction programmed by developmental hypoxia, a common complication in human pregnancy. Experiments in sheep determined in vivo fetal and adult cardiovascular function through surgical techniques not possible in humans, while those in chicken embryos isolated effects independent of maternal or placental influences. We show that hypoxia generates mitochondria-derived oxidative stress during cardiovascular development, programming endothelial dysfunction and hypertension in adult offspring. MitoQ treatment during hypoxic development protects against this cardiovascular risk via enhanced nitric oxide signaling, offering a plausible intervention strategy.
Authors
K. J. Botting, K. L. Skeffington, Y. Niu1, B. J. Allison, K. L. Brain, N. Itani, C. Beck, A. Logan4, A. J. Murray1, M. P. Murphy and A. Giussani.
Return to top of page.
| |
|
Aug 25 2020 Fetal Timeline Maternal Timeline News
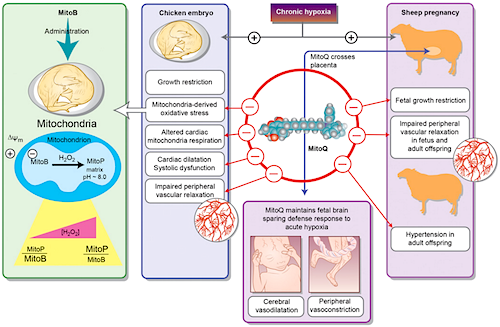 The mitochondria-targeting antioxidant MitoQ protects the hypoxic sheep fetus from cardiovascular dysfunction - which later presents as adult onset vascular dysfunction and hypertension. The MitoQ protective effect occurs without affecting the fetal brain. MitoQ effects include direct protection against mitochondria-derived oxidative stress - normalizing cardiac mitochondrial respiration in an hypoxic embryo. MitoQ treatment in sheep protects against fetal growth restriction. But, as MitoQ treatment in hypoxic chicken embryos does not change growth restriction - it suggests MitoQ protection may be due to our shared mamalian placenta. CREDIT Science Magazine.
|



