|
|
Developmental Biology - Brain Development
"Jumping" DNA Regulates Human Neurons
Virus-like elements "jump" around our genome and affect the function of our genes...
The human genome contains over 4.5 million sequences of DNA called "transposable elements". These elements are virus-like entities that "jump" around and help regulate gene expression or function. They do this by binding transcription factors, which are proteins that regulate the rate of transcription of DNA to RNA, influencing gene expression in a broad range of biological events.
Now, an international team of scientists led by Didier Trono MD PhD, at EPFL has discovered that transposable elements play a significant role in influencing the development of the human brain. This study is published in Science Advances.
His team of scientists found that these transposable elements regulate the brain's development by partnering with two specialized proteins from a family of proteins known as "Krüppel-associated box-containing zinc finger proteins", or KZFPs.
In 2019, another study led by Trono showed that KZFPs tame the regulatory activity of transposable elements in the first few days of fetal life. However, they also suspect these regulatory sequences later go on to orchestrate the development and function of adult organs.
Researchers identified that these two KZFPs are specific only to primates. They function in specific regions of the developing human and adult brains. Researchers also observed how these proteins control the activity of transposable elements - at least in those neurons and brain organoids cultured in the lab.
These two KZFPs influence the differentiation and neurotransmission profile of neurons. They guard neurons against inflammatory responses triggered if their target transposable elements were left non-functioning (meaning unexpressed).
"These results reveal how two proteins — that appeared only recently in evolution — have helped shape the human brain by facilitating the co-option of transposable elements. These virus-like entities have been remodeling our ancestral genome since the dawn of time. Our findings also suggest [K2FPs] as a possible pathogenic mechanism for diseases such as Amyotrophic Lateral Sclerosis (ALS), and other neurodegenerative/ neurodevelopmental disorders, providing leads for their prevention or treatment."
Didier Trono MD PhD, Professor of Genetics and Virology; Dean, School of Life Sciences, Swiss Institutes of Technology (EPFL), Lausanne, Switzerland.
Abstract
In the first days of embryogenesis, transposable element–embedded regulatory sequences (TEeRS) are silenced by Kruppel-associated box (KRAB) zinc finger proteins (KZFPs). Many TEeRS are subsequently co-opted in transcription networks, but how KZFPs influence this process is largely unknown. We identify ZNF417 and ZNF587 as primate-specific KZFPs repressing HERVK (human endogenous retrovirus K) and SVA (SINE-VNTR-Alu) integrants in human embryonic stem cells (ESCs). Expressed in specific regions of the human developing and adult brain, ZNF417/587 keep controlling TEeRS in ESC-derived neurons and brain organoids, secondarily influencing the differentiation and neurotransmission profile of neurons and preventing the induction of neurotoxic retroviral proteins and an interferon-like response. Thus, evolutionarily recent KZFPs and their TE targets partner up to influence human neuronal differentiation and physiology.
Authors
Priscilla Turelli, Christopher Playfoot, Dephine Grun1, Charlčne Raclot, Julien Pontis, Alexandre Coudray, Christian Thorball, Julien Duc, Eugenia V. Pankevich, Bart Deplancke, Volker Busskamp and Didier Trono.
Acknowledgements
This research was supported by National Science Foundation Graduate Research Fellowship Grant No. DGE-1144245, National Science Foundation Science and Technology Center Emergent Behavior of Integrated Cellular Systems Grant No. 3939511 and National Science Foundation Research Traineeship-Miniature Brain Machinery Grant No. 1735252. We thank Dr. Roger Kam from Massachusetts Institute of Technology who provided the transfected cell line used in this study. We thank Dr. Alvaro G. Hernández, Dr. Chris L. Wright and Dr. Christopher J. Fields from the Roy J. Carver Biotechnology Center for assistance with the production of RNA Sequencing libraries. We are also immensely grateful to our colleagues, Lauren Grant, Clare Ko, Yongdeok Kim, Stephanie López, Dr. Michael T. Hwang and Chris Seward for help in the experimentation process as well as insights during the course of this research. We appreciate discussions with Profs. Taher Saif and Martha Gillette of UIUC, and Prof. Steve Stice of University of Georgia.
Funders: This study was funded by the Canadian Institutes of Health Research. Research at The Ottawa Hospital is possible thanks to generous donations to The Ottawa Hospital Foundation. BORN Ontario is supported by the Ontario Ministry of Health and Long Term Care.
About The Ottawa Hospital
The Ottawa Hospital is one of Canada's top learning and research hospitals, where excellent care is inspired by research and driven by compassion. As the third-largest employer in Ottawa, our support staff, researchers, nurses, physicians, and volunteers never stop seeking solutions to the most complex health-care challenges. Our multi-campus hospital, affiliated with the University of Ottawa, attracts some of the most influential scientific minds from around the world. Backed by generous support from the community, we are committed to providing the world-class, compassionate care we would want for our loved ones. http://www.ohri.ca
About the University of Ottawa
The University of Ottawa is home to over 50,000 students, faculty and staff, who live, work and study in both French and English. Our campus is a crossroads of cultures and ideas, where bold minds come together to inspire game-changing ideas. We are one of Canada's top 10 research universities - our professors and researchers explore new approaches to today's challenges. One of a handful of Canadian universities ranked among the top 200 in the world, we attract exceptional thinkers and welcome diverse perspectives from across the globe. http://www.uottawa.ca.
Return to top of page.
| |
|
Sep 2 2020 Fetal Timeline Maternal Timeline News
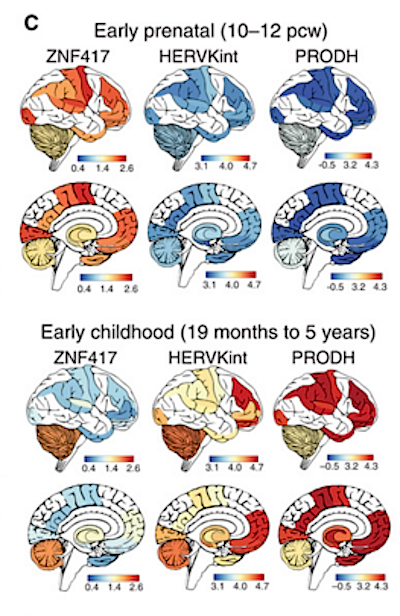 Spatial representation of ZNF417, HERVK, and PRODH genes expressed [functioning] in early prenatal and childhood brains, using RNA-seqs from the Brain Span Atlas of the Developing Human Brain. Prenatal brain is depicted as anatomically adult - just for consistency. CREDIT École polytechnique fédérale de Lausanne - EPFL.
|



