|
|
Developmental Biology - Cell Proteins
Unprecedented 3D Reconstruction of A Protein
Unprecedented detail, sets stage for developing drugs to treat diseases...
Portland State University researchers are using an advanced electron microscope to image a protein within the cell membrane, seeing it at an unprecedented level of resolution. This high resolution imaging sets the stage for developing drugs targeting that protein and treating a variety of diseases.
Reichow and his students use cryo-electron microscopy (cryo-EM) to visualize how individual cell proteins interact and function at the molecular level. They are particularly interested in a class of proteins known as membrane proteins which are key in cell to cell communication — and the target of 50% of pharmaceutical drug treatments.
The focus of their current research is connexin-46/50, two proteins from the eye lens. These proteins form pathways for cell-to-cell communication.
Using lipid nanodisc technology, researchers coax the proteins back into their original membrane environment, allowing them to see interactions between proteins at a remarkably high resolution - 1.9 Angstroms (an angstrom is one 100 millionth of a centimeter).
This team was the first to image a membrane protein below 2.0-Angstrom using cryo-EM and, at the moment, set a world record for this technology. Reichow explains that a resolution below 2.0-Angstrom is the precision desired for structure-based drug design, which uses atomic-level detail to structure the computational design of new therapeutic agents.
High resolution imaging provides new insight into how these membrane proteins interact with their native lipid environment. It also allowed scientists to view nearly 400 water molecules, which play an important role in protein structure and function.
"Drugs use water to extend their interaction with proteins. Therefore, drug manufacturers are missing a big piece of the puzzle if they don't know where water molecules are in the cell."
Steve L. Reichow PhD,
Professor, Department of Chemical Physiology and Biochemistry, Oregon Health and Science University, Portland, Oregon, USA.
This 3D structure revealed that Cx46/50 [connexin-46/50] has a long-range effect on the stability and biophysical properties of cells in the eye lens membrane, opening new doors for more exploration into how connexin channels function in their native environment.
The group's findings are published in Nature Communications.
Abstract
Gap junctions establish direct pathways for cells to transfer metabolic and electrical messages. The local lipid environment is known to affect the structure, stability and intercellular channel activity of gap junctions; however, the molecular basis for these effects remains unknown. Here, we incorporate native connexin-46/50 (Cx46/50) intercellular channels into a dual lipid nanodisc system, mimicking a native cell-to-cell junction. Structural characterization by CryoEM reveals a lipid-induced stabilization to the channel, resulting in a 3D reconstruction at 1.9 å resolution. Together with all-atom molecular dynamics simulations, it is shown that Cx46/50 in turn imparts long-range stabilization to the dynamic local lipid environment that is specific to the extracellular lipid leaflet. In addition, ~400 water molecules are resolved in the CryoEM map, localized throughout the intercellular permeation pathway and contributing to the channel architecture. These results illustrate how the aqueous-lipid environment is integrated with the architectural stability, structure and function of gap junction communication channels.
Authors
Jonathan A. Flores, Bassam G. Haddad, Kimberly A. Dolan, Janette B. Myers, Craig C. Yoshioka, Jeremy Copperman, Daniel M. Zuckerman and Steve L. Reichow.
Kimberly A. Dolan
Present address: Biophysics Graduate Group, University of California, Berkeley, CA, 94720, USA
Affiliations
Department of Chemical Physiology and Biochemistry, Oregon Health and Science University, Portland, OR, 97239, USA/Jonathan A. Flores & Steve L. Reichow.
Department of Chemistry, Portland State University, Portland, OR, 97201, USA/Bassam G. Haddad, Kimberly A. Dolan, Janette B. Myers & Steve L. Reichow.
Department of Biomedical Engineering, Oregon Health and Science University, Portland, OR, 97239, USA/Craig C. Yoshioka, Jeremy Copperman & Daniel M. Zuckerman.
These authors contributed equally: Jonathan A. Flores, Bassam G. Haddad, Kimberly A. Dolan.
Acknowledgements
The authors thank Dror Chorev and Carol V. Robinson for help analyzing specimens prepared for CryoEM. We are grateful to the staff at the OHSU Multiscale Microscopy Core and Advanced Computing center, and to the Pacific Northwest Center for CryoEM (supported by NIH Grant U24GM129547), and accessed through EMSL (grid.436923.9). J.A.F. and B.G.H. are supported by the National Institutes of Health NRSA fellowships F31-EY030409 and F31-EY031580, respectively. K.A.D. is supported by National Institutes of Health BUILD EXITO Program (TL4-GM118965) and Berkeley Molecular Biophysics Training Grant (T32-GM008295). C.C.Y. is supported by OHSU. J.C. and D.M.Z. are supported by the OHSU Center for Spatial Systems Biomedicine, by the National Science Foundation (MCB 1715823), and by the National Institutes of Health (R01-GM115805). S.L.R. is supported by the National Institutes of Health (R35-GM124779).
Return to top of page.
| |
|
Sep 3 2020 Fetal Timeline Maternal Timeline News
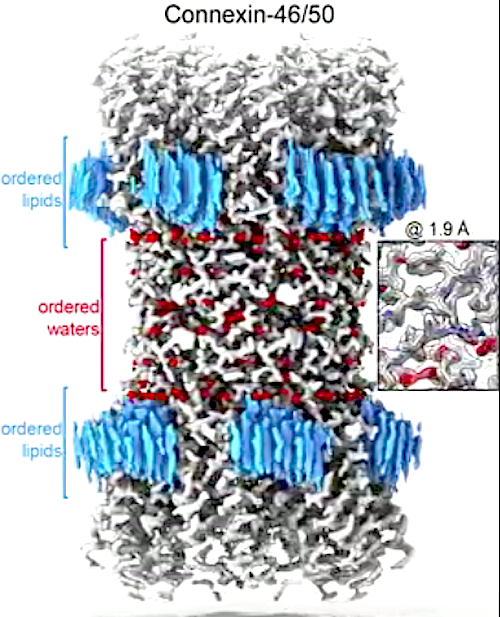 The structure of cell-to-cell communication channels (Connexin-46/50) at 1.9 Å resolution captured by the Reichow Lab, using electron cryo-microscopy (CryoEM). This work provides a view of WHITE communication channels, as well as ordered RED water molecules and the BLUE surrounding lipids — at near-atomic level detail for the first time. CREDIT Reichow Lab | Portland State University. MOVIE
|



