|
|
Developmental Biology - Pediatric Dysphagia
What Causes Swallowing Disorders?
Misfiring brain cells may cause spontaneous impulses not to be in balance with inhibiting responses...
Fralin Biomedical Research Institute scientists have identified the molecular roots of a swallowing disorder in DiGeorge Syndrome.
Misfiring brain cells that control key parts of our mouth and tongue may be creating swallowing difficulties in children with this genetic neuro-developmental disorder. This new insight is according to neuroscientists with Virginia Tech and George Washington University.
Research conducted with a mouse model of DiGeorge syndrome, has revealed brain motor neuron cells fire out of synch with the mechanisms expected to control their function.
Identifying ways to calm motor neurons responsible for moving the tongue could lead to improved function in very young children with the disorder. DiGeorge Syndrome patients have difficulty swallowing, eating, or making sounds. Despite this latest observation, scientists believe more research is needed before therapies can be developed.
"We are continuing to make the case that activity of motor neurons that command movement of key parts of the mouth, tongue, and pharynx are disrupted by the same mechanisms causing genetic neurodevelopmental disorders. Our goal is to learn about the causes of these symptoms to help children as early in life as possible."
Anthony-Samuel LaMantia PhD, Developmental Neurobiologist, Professor, Department of Biological Sciences of the College of Science, VTC and senior co-author of the study; Lamantia also heads the Center for Neurobiology Research at the Fralin Biomedical Research Institute.
The study was published online in advance in eNeuro, an open-access journal of the Society for Neuroscience.
Problems ingesting, chewing, or swallowing food occur in up to 80% of most children with developmental disorders, which can lead to food aspiration, choking, or life-threatening respiratory infections.
Suckling, feeding, and swallowing difficulties - known as pediatric dysphagia - are among the most serious and frequent complications in infants. They are common in DiGeorge syndrome, a genetic disease caused when a small part of chromosome 22 is missing. The syndrome carries a high risk for autism spectrum disorder and schizophrenia, as well as heart, face, and limb malformations.
Associate research professor Xin Wang and co-senior author David Mendelowitz, vice chair and professor of pharmacology and physiology, both with the George Washington University School of Medicine and Health Sciences, traced motor neurons in mouse models of DiGeorge syndrome. Each muscle and motor neuron was labeled and tracked back to its target muscle, and its electric properties were recorded.
Motor neurons responsible for forward and backward movement of the tongue in DiGeorge syndrome models, spontaneously fire compared to motor neurons in normal mice. Their excitatory impulses are not balanced by inhibitory responses.
As a result, the increased excitability of motor neurons affected compression and movement of tongue muscles, which would threaten both food intake efficiency and airway safety in infants and toddlers.
Abstract
We asked whether the physiological and morphological properties of hypoglossal motor neurons (CNXII MNs) that innervate protruder or retractor tongue muscles are disrupted in neonatal LgDel mice that carry a heterozygous deletion parallel to that associated with DiGeorge/22q11.2. Deletion Syndrome (22q11.2DS). Disrupted coordination of tongue movement in LgDel mouse pups may contribute to suckling, feeding and swallowing (S/F/S) disruptions that parallel pediatric dysphagia in infants and toddlers with 22q11.2DS. Utilizing an in vitro rhythmically active medullary slice preparation we found spontaneous firing as well as inhibitory post-synaptic current (IPSC) frequency differed significantly in neonatal LgDel versus WT protruder and refractor CNXII MNs that were identified by retrograde tracing from their target muscles. In response to respiration-related activity, initation and decay of transiently increased firing in WT protruder MNs is delayed in LgDel, accompanied by altered excitatory/inhibitoty {E/I) balance. In addition, LgDel retractor MNs have a transient increase in firing with diminished IPSC frequency that is not seen in WT. There were no significant differences in cell body volume of either XII class in WT and LgDel. Sholl analysis showed the total numbers of dendritic intersections (at 50 and 90 um radii from the cell soma) were significantly greater for LgDei versus WT retractor MNs. Thus, the physiological, synaptic and cellular properties of distinct classes of CNXII MNs that coordinate tongue movement in neonatal WT mice are altered in LgDel. Such changes could contribute to sub-optimal coordination of S/F/S that underlies pediatic dysphagia in 22q11.2DS.
Significance Statement
Pediatric dysphagia is a frequent and serious clinical complication in up to 85% clinically defined neurodevelopmental disorders, including DiGeorge/22q11.2 Deletion Syndrome. Heterozygous deletion of the full set of mouse orthologues of human 22q11 genes disrupts key physiological properties of distinct classes of hypoglossal motor neurons that coordinate oro-pharyngeal function in the LgDel mouse model of 22q11DS, the only established genetic model for pediatric dysphagia. We found significant differences in firing activity, synaptic inputs and morphology when comparing protruder and retractor CNXII MNs from LgDel and WT animals.
Our observations provide a foundation for understanding infant hypoglossal nerve as well as tongue function or dysfunction, and their contribution to perinatal feeding and swallowing abnormalities in 22q11.2DS.
Authors
Xin Wang, Anastas Popratiloff, Zahra Motahari, Anthony-Samuel La Mantia and David Mendelowitz.
Acknowledgements
Funders: This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
Return to top of page.
| |
|
Sep 7 2020 Fetal Timeline Maternal Timeline News
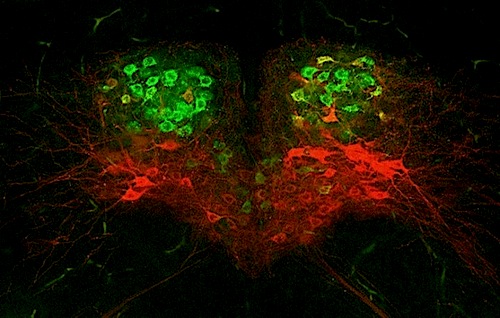 BrainStem of a newborn mouse: motor neurons that retract the tongue GREEN, neurons that protrude the tongue RED. Activity of these two populations of motor neurons is not coordinated in mice with the mutation causing DiGeorge Syndrome. Lack of coordination likely underlies sucking, feeding, and swallowing difficulty in mice, and perhaps human infants with the disorder. It occurs when a piece of the 22nd chromosome is missing. CREDIT Xin Wang & Anastas Popratiloff.
|



