|
|
Developmental Biology - Brain Glymphatic System
Circadian Rhythms "Wash" Waste From Brain
People who rely on sleeping during daytime hours are at greater risk for developing neurological disorders...
New research details how a complex set of molecular and fluid dynamics make up our glymphatic system - a unique process for vetebrate brain cell waste removal. This process synchronizes with our master internal clock to regulate our sleep-wake cycle, suggesting people who rely on sleep during daytime hours are at greater risk for developing neurological disorders.
"These findings show that glymphatic system function is not solely based on sleep or wakefulness, but by daily rhythms dictated by our biological clock."
Maiken Nedergaard MD, DM Sc, neuroscientist and co-director, Center for Translational Neuromedicine, University of Rochester Medical Center (URMC), and senior author of the study appearing in Nature Communications.
The brain's self-contained waste removal process was first discovered in 2012 by researchers in the Nedergaard lab. The system consists of a network of plumbing that follows the path of blood vessels and cerebrospinal fluid (CSF) pumps throughout our brain tissue. While the system washes away waste, researchers a few years after its initial discovery found that it primarily functions as we sleep.
Since these initial discoveries, Nedergaard's lab and others have shown how blood pressure, heart rate, circadian timing and depth of sleep - share in glymphatic function and chemical signaling that turns the system on and off.
They also showed disrupted sleep or trauma can cause the system to break down, allowing toxic proteins to accumulate in the brain. This could give rise to neurodegenerative diseases such as Alzheimer's.
Links between circadian rhythms and our glymphatic system is the subject of this newest paper. Circadian rhythm - a 24-hour internal clock regulating important bodily functions, including the sleep-wake cycle - are maintained in a small brain area called the suprachiasmatic nucleus.
In this newest study conducted in mice, researchers show that even when mice were anesthetized during daylight hours, their glymphatic system still functioned along their typical rest period opposite that of humans - mice are nocturnal.
"Circadian rhythms in humans are also tuned to a day-wake, night-sleep cycle. Because this timing also influences our glymphatic system, these findings suggest people who rely on cat naps during the day to catch up on sleep — or work during a night shift, may be at risk for developing neurological disorders.
In fact, clinical research shows individuals who rely on sleeping during daytime hours are at much greater risk for Alzheimer's and dementia along with other health problems."
Lauren Hablitz PhD, Assistant Professor, URMC Center for Translational Neuromedicine and first author of the study.
Astrocytes play multiple functions in the brain. Observations from this study lead researchers to believe astrocytes in the suprachiasmatic nucleus may help regulate circadian rhythms. Astrocytes serve as gatekeepers controlling the flow of CSF throughout our central nervous system. Study results [however] suggest communication between astrocytes in different parts of the brain may be optimized by our glymphatic system's function during sleep.
During wakefulness, researchers found the glymphatic system diverts CSF to lymph nodes in the neck. As lymph nodes act as waystations regulating our immune system, research suggests CSF may represent a "fluid clock" helping wake up the body's infection fighting capability during the day.
"Establishing a role for communication between astrocytes and the significant impacts of circadian timing on glymphatic clearance dynamics represent a major step in understanding the fundamental process of waste clearance regulation in the brain. This knowledge is crucial to developing future countermeasures that offset the deleterious effects of sleep deprivation and addresses future multi-domain military operation requirements for Soldiers to sustain performance over longer periods without the ability to rest."
Frederick Gregory PhD, Program Manager, Army Research Office, U.S. Army Combat Capabilities Development Command's Army Research Laboratory — which helped fund this research.
Abstract
The glymphatic system is a network of perivascular spaces that promotes movement of cerebrospinal fluid (CSF) into the brain and clearance of metabolic waste. This fluid transport system is supported by the water channel aquaporin-4 (AQP4) localized to vascular endfeet of astrocytes. The glymphatic system is more effective during sleep, but whether sleep timing promotes glymphatic function remains unknown. We here show glymphatic influx and clearance exhibit endogenous, circadian rhythms peaking during the mid-rest phase of mice. Drainage of CSF from the cisterna magna to the lymph nodes exhibits daily variation opposite to glymphatic influx, suggesting distribution of CSF throughout the animal depends on time-of-day. The perivascular polarization of AQP4 is highest during the rest phase and loss of AQP4 eliminates the day-night difference in both glymphatic influx and drainage to the lymph nodes. We conclude that CSF distribution is under circadian control and that AQP4 supports this rhythm.
Authors
Lauren M. Hablitz, Virginia Plá, Michael Giannetto, Hanna S. Vinitsky, Frederik Filip Stæger, Tanner Metcalfe, Rebecca Nguyen, Abdellatif Benrais and Maiken Nedergaard.
Acknowledgements
The authors thank Dan Xue for graphic illustrations and animations. Funding: United States Department of Defense | United States Army | U.S. Army Research, Development and Engineering Command | Army Research Office (ARO) - MURI W911NF1910280 [Nedergaard]. U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS) - R01NS100366 [Nedergaard]. U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS) - RF1AG057575 [Nedergaard]. Fondation Leducq Transatlantic Networks of Excellence Program, Novo Nordisk and Lundbeck Foundations, and the EU Horizon 2020 research and innovation program (grant no. 666881; SVDs@target) [Nedergaard].
The authors declare no competing interests.
Return to top of page.
| |
|
Sep 10 2020 Fetal Timeline Maternal Timeline News
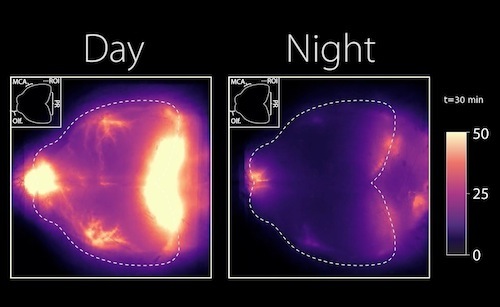 Day to night images reflect lymph and nerve activity in mouse brain. CREDIT The Authors.
|



