|
|
Developmental Biology - Brain Oxygen Levels
Estrogen Key Protection Against Stroke Brain Damage
When our brain isn't getting enough oxygen, estrogen in both males and females hyperactivates astrocyte cells, increasing their numbers to protect brain function...
With stroke or other brain injury, low brain oxygen levels can occur. Star-shaped brain cells called astrocytes, help bring the brain back to order. Astrocystes regularly provide fuel and support to neurons. But, as reported in the Journal of Neuroscience, should brain cells become "highly reactive" and increase signaling other cells, astrocytes release neuroprotective factors to clear neurotoxins. Astrocytes produce protective estrogen, but neurons produce estrogen critical in this protective cascade.
"Astrocytes are always there, hovering and supporting. When something bad happens, they are supposed to go into overdrive and get big and pushy. But, our research suggests you must have neural estrogen for that to happen."
Darrell W. Brann PhD,
Department of Neuroscience and Regenerative Medicine, Medical College of Georgia, Augusta, Georgia, USA.
The research appears to be the first to demonstrate how neuron derived estrogen is critical to astrocyte activation following ischemic injury, such as stroke. Scientists reasoned that understanding how estrogen is controlled, will unlock therapeutic targets to one day help regulate estrogen's protection of the brain not only in the face of injury, but also during normal aging.
"The hallmark of activation: astrocytes get very large and hypertrophic. Their volume increases tremendously, often more than doubling in size, although there is no evidence they increase in number."
Darrell W. Brann PhD
To understand why and how astrocytes do this, researchers knocked out the enzyme aromatase in model animals (referred to as: knockout mice). Aromatase is critical to estrogen production in neurons of the forebrain, the largest human brain region. They identified how estrogen protects the brain by suppressing fibroblast growth factor (FGF2) during ischemia (stroke). FGF2 is known to suppress astrocyte activation.
When using a neutralizing antibody to block FGF2, astrocytes became more active and decreased neuron damage. Giving more estrogen produced similar benefits, including improving cognition after ischemia.
But, without the ability to make aromatase - and consequently estrogen - researchers found activation of astrocytes significantly reduced in male and female neurons. Brain estrogen levels decreased with worse damage and more cognitive dysfunction. Looking closer, researchers found changes in pathways and genes associated with astrocyte activation, brain inflammation and oxidative stress in the aromatase knockout mice. They also saw less neuroprotective growth factors, such as brain derived neurotrophic factor and insulin-like growth factor 1, typically released at increased rates by astrocytes in response to ischemia. Yet, they did see more suppressive substances such as the brake: FGF2.
These findings are more evidence that decreased activation of astrocytes has functional consequences on neuroprotection.
"We know estrogen can just leave the astrocyte and go to the neuron and help regulate protection. It also can help regulate microglia, another type of glial cell in the brain (astrocytes are also glial cells) that - instead - release inflammatory factors exacerbating damage."
Darrell W. Brann PhD
Activated astrocytes help clear glutamate, the brain's most abundant excitatory neurotransmitter, which normally helps neurons communicate. But, without estrogen from neurons, glutamate transporter GLT-1 which removes about 90% of glutamate is significantly decreased. It can then accumulate at toxic levels in the brain to become a major cause of neuron destruction. "Glutamate is essential for brain function. But overproduced it's brain toxic," Brann adds.
When all is going well in the brain, only neurons appear to be a local estrogen source, but with a stressor like ischemia, aromatase and the estrogen it enables are known to increase significantly in astrocytes as well.
"We know that you have to have astrocyte activation to have aromatase activated. We only see aromatase in astrocytes when they are activated after injury - after stress. Aromatase increases when astrocytes start making estrogen. That did not happen in the knockout mice."
Darrell W. Brann PhD
Now researchers want to learn why astrocytes don't routinely produce aromatase and its resulting estrogen and exactly why they do produce it during ischemia. Researchers think these answers will provide more direction for possible intervention, for men and women, with oxygen challenges to their brains. Brann notes there are other compounds that produce estrogen in the brain that the team will be working with moving forward.
Brann's lab reported last year in The Journal of Neuroscience that estrogen in the brain is important for neuron communication and making memories. When male and female neurons don't make estrogen, they have less dense spines and synaptic exchanges. Direct evidence that neuron-derived estrogen is critical in brain function.
While these studies found neuroprotection was essentially equal between males and females - Brann's lab did find significant impact, about 90% versus 60%, between female and male memory. Also, blocking FGF2 signaling by injecting FGFR3 (a neutralizing antibody) reversed diminished neuroprotective astrocyte reactivity, and reduced the effect of neuronal damage in FBN-ARO-KO mice. Additionally, E2 replacement in living mice suppressed FGF2 signaling, and rescued compromised cognitive deficits in affected mice. The research data provided genetic evidence for using neuron-derived E2 in astrocyte activation, neuroprotection and cognitive preservation following ischemic injury to the brain.
Ischemic strokes account for 87% of strokes, according to the American Heart Association. And, just like circulating estrogen levels in females, brain-derived estrogen decreases with age in both sexes.
Darrell W. Brann PhD
Abstract
17฿ Brain-estradiol (E2) is produced from androgens via the action of the enzyme aromatase. E2 is known to be made in neurons in the brain, but the functions of neuron-derived E2 in the ischemic brain are unclear. Here, we used a forebrain neuron-specific aromatase knockout (FBN-ARO-KO) mouse model to deplete neuron-derived E2 in the forebrain and determine its roles after global cerebral ischemia (GCI). We demonstrated that ovariectomized female FBN-ARO-KO mice exhibited significantly attenuated astrocyte activation, astrocytic aromatization and decreased hippocampal E2 levels, as compared to FLOX mice. Furthermore, FBN-ARO-KO mice had exacerbated neuronal damage and worse cognitive dysfunction after GCI. Similar results were observed in intact male mice. RNA-seq analysis revealed alterations in pathways and genes associated with astrocyte activation, neuroinflammation and oxidative stress in FBN-ARO-KO mice. The compromised astrocyte activation in FBN-ARO-KO mice was associated with robust downregulation of the astrocyte-derived neurotrophic factors, BDNF and IGF-1, as well as the astrocytic glutamate transporter, GLT-1. Neuronal FGF2, which acts in a paracrine manner to suppress astrocyte activation, was increased in FBN-ARO-KO neurons. Interestingly, blocking FGF2 signaling by central injection of FGFR3 neutralizing antibody was able to reverse the diminishment in neuroprotective astrocyte reactivity, and attenuate neuronal damage in FBN-ARO-KO mice. Moreover, in vivo E2 replacement suppressed FGF2 signaling, and rescued the compromised reactive astrogliosis and cognitive deficits. Collectively, our data provide novel genetic evidence for a beneficial role of neuron-derived E2 in astrocyte activation, neuroprotection and cognitive preservation following ischemic injury to the brain.
Significance Statement
Following cerebral ischemia, astrocytes become highly reactive and can exert neuroprotection through the release of neurotrophic factors and clearance of neurotoxic glutamate. The current study advances our understanding of this process by demonstrating that neuron-derived 17฿-estradiol (E2) is neuroprotective and critical for induction of reactive astrocytes and their ability to produce astrocyte-derived neurotrophic factors, BDNF and IGF-1, and the glutamate transporter, GLT-1 after ischemic brain damage. These beneficial effects of neuronal-derived E2 appear to be due, at least in part, to suppression of neuronal FGF2 signaling, which is a known suppressor of astrocyte activation. These findings suggest that neuronal-derived E2 is neuroprotective after ischemic brain injury via a mechanism that involves suppression of neuronal FGF2 signaling, thereby facilitating astrocyte activation.
Authors
Yujiao Lu, Gangadhara R. Sareddy, Jing Wang, Quanguang Zhang, Fu-Lei Tang, Uday P. Pratap, Rajeshwar R. Tekmal, Ratna K. Vadlamudi and Darrell W. Brann.
Acknowledgements
The authors declare no competing financial interests.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
Brann along with Dr. Ratna Vadlamudi, Molecular Biologist, Professor of Obstetrics and Gynecology, University of Texas Health, San Antonio, are Co-corresponding Authors of the study and Co-principal Investigators on the National Institute of Neurological Disorders and Stroke grant funding the research (Grant R01NS088058).
Return to top of page.
| |
|
Sep 11 2020 Fetal Timeline Maternal Timeline News
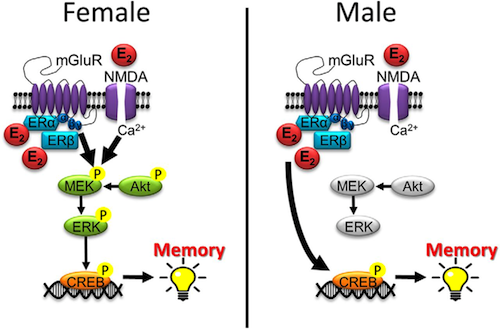 The steroid hormone 17B-estradiol is our main human estrogen. It is not only involved in sexual maturation and reproduction but also has a myriad of important roles throughout the body, for example, affecting the cardiovascular system, lipid metabolism and brain health. Therefore,it cannot be considered as only a female hormone. CREDIT Wendy Koss ENeuro.
|



