|
|
Developmental Biology - Shape of the Early Embryo
Embryos Take Shape Under Pressure
Scientists demonstrate how embryo structures deform under compression, a phenomenon that may determine the development of body parts...
The embryo of an animal first looks like a hollow sphere. But, through the external pressures of quickly forming new cells, older cells come under pressure and are pushed and folded back on themselves to form cavities or pouches (invagination). Through continuous cellular expansion, different body structures such as brain, digestive tract, heart etc. form from the internal pressure of an expanding mass of cells. Folding into organs, tissues and bone.
According to a hypothesis dating back more than a century — buckling under pressure may be the dominant mechanism triggering invagination. Any material can be deformed under the compression of increasing mass.
Although this theory has long had the support of biologists, there has never been formal proof. Mainly due to the difficulty of measuring the tiny forces involved.
Finally, thanks to a study carried out by a multidisciplinary team of scientists from the University of Geneva (UNIGE) and published in the journal Developmental Cell — new evidence substantiates the concept. It is the result of collaborations between specialists in biological experimentation, analytical theoretical physics and computer simulation.
"The basic question underpinning our work is how to shape cellular tissue. This mechanism is not powerful enough alone to explain major invaginations appearing during the development of the blastocyst (an early stage embryo)."
Aurélien Roux PhD, Professor, Department of Biochemistry; National Center of Competence in Research Chemical Biology, University of Geneva, UNIGE's Faculty of Science, Geneva, Switzerland.
Observing embryo development makes it possible to describe several mechanisms at work. One is apical constriction - or curvature of the surface of the embryo - the result of a deformation in the path of cells (beginning with the "apex" tightening and the "base" relaxing).
"A century ago, biologists suggested 'buckling' is the mechanism that generates deep folds. The same mechanism is observed when you flatten a sheet of paper and bring it's two opposite edges together — the middle of the sheet rises (buckles).
In embryos, lateral force comes from the proliferation of cells which exert increasing pressure on the surface of a sheet of cells. This surface is confined into a vitelline envelope (that transparent casing enclosing an egg yolk) — which, although elastic, prevents spatial expansion."
Aurélien Roux PhD
This explanation easily won consensus among biologists. It was long believed unthinkable to measure surface tension on an embryo in order to verify buckling (which obeys the well-known laws of material physics).
Analytical, IT & Biological Approaches
Geneva scientists needed quantitative proof of buckling, and so conducted a study. Anastasiya Trushko PhD, Professor, Department of Biochemistry, manufactured small envelopes with the physical properties of natural vitelline, succeeding in growing a monolayer of one hundred cells on inner envelope surfaces. These small embryomodels, less than half a millimetre in diameter, were controlled under laboratory conditions to recreate cell invagination and observed microscopically. Mechanical invagination forces were measured thanks to small variations in thickness of each artificial embryo.
Meanwhile Carles Blanch-Mercader, biochemist, along with professor Karsten Kruse, UNIGE Department of Theoretical Physics, used these measurements to reveal relationships between strength and shape of buckling in the artificial embryos. Material physics equations helped them extract the macroscopic mechanical parameters of cellular tissues, such as those for surface stiffness. In order to link these macroscopic characteristics to biological processes at the cell level, Aziza Merzouki along with Bastien Chopard, computer simulated embryo development as a set of independent cell interactions.
"By quantifying buckling as precisely as possible, we were able to demonstrate that it is a potential mechanism for explaining invagination in embryos. It is likely that other mechanisms, such as 'apical constriction', initiates 'folding' and that 'buckling' accentuates it before finally creating the expected result."
Bastien Chopard PhD, Department of Computer Science, University of Geneva, Switzerland.
Significance Statement
Highlights
• A proliferating epithelium encapsulated in a hollow sphere spontaneously invaginates
• Epithelial proliferation generates compressive stresses that deform the elastic shell
• Coupling between stress and folding shape shows that folding arises from buckling
• Epithelial elastic moduli are inferred from buckling theory and experiments.
Summary
Many organs are formed through folding of an epithelium. This change in shape is usually attributed to tissue heterogeneities, for example, local apical contraction. In contrast, compressive stresses have been proposed to fold a homogeneous epithelium by buckling. While buckling is an appealing mechanism, demonstrating that it underlies folding requires measurement of the stress field and the material properties of the tissue, which are currently inaccessible in vivo. Here, we show that monolayers of identical cells proliferating on the inner surface of elastic spherical shells can spontaneously fold. By measuring the elastic deformation of the shell, we infer the forces acting within the monolayer and its elastic modulus. Using analytical and numerical theories linking forces to shape, we find that buckling quantitatively accounts for the shape changes of our monolayers. Our study shows that forces arising from epithelial growth in three-dimensional confinement are sufficient to drive folding by buckling.
Authors
Anastasiya Trushko, Ilaria Di Meglio, Aziza Merzouki, Karsten Kruse, Bastien Chopard and Aurélien Roux.
Acknowledgements
The authors thank Oresti Malaspinas for his useful insights into the project.
Funding
A.R. and B.C. acknowledge funding from the SystemsX EpiPhysX consortium . A.R. acknowledges funding from Human Frontier Science Program Young Investigator grant RGY0076/2009-C ; the Swiss National Fund for Research grants nos. 31003A_130520 , 31003A_149975 , and 31003A_173087 ; and the European Research Council Consolidator grant no. 311536 . I.D.M. and A.R. acknowledge funding from Secrétariat d’Etat à la Recherche et à l’innovation grant agreement REF-1131-52107 . I.D.M., S.A., A.R., and J.G. acknowledge funding from the EU Horizon2020 Marie Sklodowska-Curie ITN “BIOPOL” (grant agreement no. 641639
Declaration of Interests
The authors declare no competing interests.
Return to top of page.
| |
|
Sep 16 2020 Fetal Timeline Maternal Timeline News
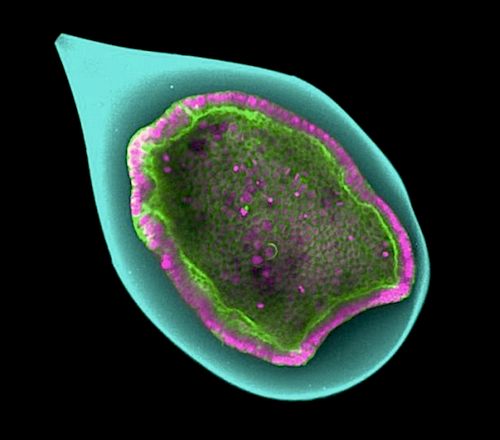 A hundred cells (pink and green) encapsulate into a hollow sphere (cyan), and form an epithelia cell layer which invaginates spontaneously. CREDIT @UNIGE/Aurélien Roux.
|



