|
|
Developmental Biology - Congenital Gut Malabsorption
Hormone Reverses Infant Gut Malabsorption
Human intestinal organoids grown from stem cells, model congenital malabsorption disorder in babies...
Scientists at Cincinnati Children's used human intestinal organoids grown from stem cells to discover how our bodies control the absorption of nutrients from the food we eat. They further found that one hormone might be able to reverse a congenital disorder in babies who cannot adequately absorb nutrients and need intravenous feeding to survive.
Heather A. McCauley PhD, research associate at Cincinnati Children's Hospital Medical Center, found that the hormone peptide YY, also called PYY, can reverse congenital malabsorption in mice. With a single PYY injection per day, 80% of the mice survived. Normally, only 20% to 30% survive.
This indicates PYY might be a possible therapeutic for people with severe malabsorption.
Researchers point out that poor absorption of macronutrients is a global health concern, underlying ailments such as malnutrition, intestinal infections and short-gut syndrome. So, identification of factors regulating nutrient absorption has significant therapeutic potential.
McCauley is lead author of a manuscript published Sept. 22 in Nature Communications, which reports the absorption of nutrients - in particular, carbohydrates and proteins - is controlled by enteroendocrine cells within the gastrointestinal tract.
Babies born without enteroendocrine cells - or with non-functioning enteroendocrine cells - have severe malabsorption and require IV nutrition.
"This study allowed us to understand how important this one rare cell type is in controlling how the intestine absorbs nutrients and functions on a daily basis."
Heather A. McCauley PhD, Division of Developmental Biology and Center for Stem Cell and Organoid Medicine, Cincinnati Children?s Hospital Medical Center, Cincinnati, Ohio, USA.
The Cincinnati Children's study was the first to describe a mechanism linking enteroendocrine cells to the absorption of macronutrients like carbohydrates and amino acids.
One key finding is how these cells, upon sensing ingested nutrients, prepare the intestine to absorb nutrients through control of influx and outflux of electrolytes and water. Absorption of carbohydrates and protein is then linked to the movement of ions in the intestine.
The scientists relied on human intestinal organoid models created in the lab, explains James A. Wells PhD, Director, Basic Research and an Allen Foundation Distinguished Investigator at Cincinnati Children's Hospital.
Grown from stem cells, organoids are small formations of human organ that have an architecture and functions that are similar to their full-size counterparts.
Cincinnati Children's launched efforts to make organoids from human pluripotent stem cells in 2006, explains Wells. The malabsorption study used three different human small intestinal tissue models - all derived from pluripotent stem cells ? which can form any kind of tissue in the body.
"This study highlights decades of basic research into how organs are made and how they function, now leading to breakthroughs. Human organoids are essentially a much more realistic avatar to patients with these rare mutations. They allow us to model much more faithfully the human disease."
James A. Wells PhD, Divisions of Developmental Biology and Endocrinology, Center for Stem Cell and Organoid Medicine, Cincinnati Children?s Hospital Medical Center, Cincinnati, Ohio, USA.
Abstract
Significance
Influenza infection during pregnancy is associated with increased maternal and perinatal complications. Here, we show that, during pregnancy, influenza infection leads to viral dissemination into the aorta, resulting in a peripheral ?vascular storm? characterized by enhanced inflammatory mediators; the influx of Ly6C monocytes, neutrophils, and T cells; and impaired vascular function. The ensuing vascular storm induced hypoxia in the placenta and fetal brain and caused an increase in circulating cell free fetal DNA and soluble Flt1 release. We demonstrate that vascular dysfunction occurs in response to viral infection during pregnancy, which may explain the high rates of morbidity and mortality in pregnant dams, as well as the downstream perinatal complications associated with influenza infection.
Abstract
The ability to absorb ingested nutrients is an essential function of all metazoans and utilizes a wide array of nutrient transporters found on the absorptive enterocytes of the small intestine. A unique population of patients has previously been identified with severe congenital malabsorptive diarrhea upon ingestion of any enteral nutrition. The intestines of these patients are macroscopically normal, but lack enteroendocrine cells (EECs), suggesting an essential role for this rare population of nutrient-sensing cells in regulating macronutrient absorption. Here, we use human and mouse models of EEC deficiency to identify an unappreciated role for the EEC hormone peptide YY in regulating ion-coupled absorption of glucose and dipeptides. We find that peptide YY is required in the small intestine to maintain normal electrophysiology in the presence of vasoactive intestinal polypeptide, a potent stimulator of ion secretion classically produced by enteric neurons. Administration of peptide YY to EEC-deficient mice restores normal electrophysiology, improves glucose and peptide absorption, diminishes diarrhea and rescues postnatal survival. These data suggest that peptide YY is a key regulator of macronutrient absorption in the small intestine and may be a viable therapeutic option to treat patients with electrolyte imbalance and nutrient malabsorption.
Authors
Heather A. McCauley, Andrea L. Matthis, Jacob R. Enriquez, Jonah T. Nichol, J. Guillermo Sanchez, William J. Stone, Nambirajan Sundaram, Michael A. Helmrath, Marshall H. Montrose, Eitaro Aihara and James M. Wells.
AcknowledgementsScience
The study was supported by grants from the National Institutes of Health (U19 AI116491, P01 HD093363, UG3 DK119982, U01 DK103117); S&R Foundation and American Physiological Society; the American Diabetes Association (1-17-PDF-102); the Shipley Foundation and the Allen Foundation. Support was also received from the Digestive Disease Research Center (P30 DK078392).
Return to top of page.
| |
|
Sep 24 2020 Fetal Timeline Maternal Timeline News
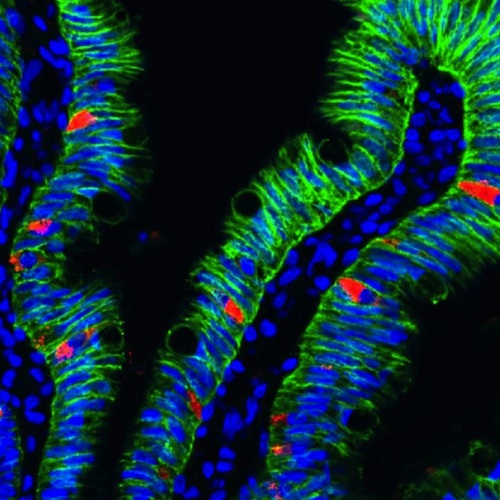 Image of human intestinal organoids with enteroendocrine cells (RED) embedded within the intestinal cells of Human Intestinal Organoids or HIOs (GREEN). CREDIT Heather A. McCauley, PhD/Cincinnati Children's Hospital.
|



