|
|
Developmental Biology - Maternal Metabolites Activate Fetal Neural Genes
Mom's Intestinal Microbes Affect Fetal Brain
In mice, maternal gut microbiata regulate which genes activate in the growing fetal brain...
UCLA research reveals during pregnancy in mice, billions of bacteria and other microbes living in a mother's intestines regulate key small molecules (metabolites) important for healthy fetal brain development. The research was published September 23 in the journal Nature.
While maternal gut microbiota have previously been associated with abnormalities in mouse pup brain function and behavior, often in response to (1) infection, (2) high-fat diet or (3) stress during pregnancy, scientists did not know how gut microbes influence brain development during critical prenatal periods.
To test the impact of no gut microbiata on metabolites and other maternal biochemicals circulating in maternal blood to nurture the rapidly developing fetal brains, researchers treated mouse food with antibiotics (to kill off maternal gut bacteria) and bred microbe-free mice in the lab.
"Depleting the maternal gut microbiota, using both methods, similarly disrupted fetal brain development. Depleting maternal gut microbiota also altered which genes became active in the brains of developing offspring."
Helen Vuong, postdoctoral scholar, Elaine Hsiao laboratory, University of California Los Angeles (UCLA) and lead author.
Researchers found many genes affected were involved in forming new axons, the tiny fibers on neuron ends which link brain cells and enable cells to signal each other through electrical impulses. Particularly reduced in number and length were axons connecting the brain's thalamus to the brain cortex. These axons are particularly important as they sense the environment. Consistent with this, pups from mothers lacking gut microbiota had impaired sensory behaviors. Such findings indicate maternal gut microbiota promote healthy fetal brain development.
Vuong: "When we measured the types and levels of molecules in the maternal blood, fetal blood and fetal brain, we found that particular metabolites were commonly decreased or missing when the mother was lacking a gut microbiota during pregnancy."
Biologists then grew neurons in the presence of these key metabolites. Also reintroducing missing metabolites into the microbiata-depleted pregnant mice.
"When we grew neurons in the presence of these metabolites, they developed longer axons and a greater number of axons. And when we supplemented pregnant mouse diets with key metabolites that had been decreased or eliminated, we saw restored levels of those metabolites in fetal brains and prevention of impaired behavior and improved axon development.
Gut microbiota has the incredible capability to regulate many biochemicals not only in the pregnant mother but also in the developing fetus and fetal brain. Our findings also pinpoint select metabolites that promote axon growth."
Helen E. Vuong PhD
These results suggest interactions between microbiota and nervous system begin prenatally through influence by maternal gut microbiota on fetal brain — at least in mice. However, the applicability of these findings to humans is still unclear, according to Elaine Hsiao PhD, Associate Professor, integrative biology, physiology, microbiology, immunology and molecular genetics, UCLA.
"We don't know whether and how these findings apply to humans. However, many neuro-developmental disorders are believed to be caused by both genetic and environmental risk factors experienced during pregnancy. Our study suggests maternal gut microbiota during pregnancy should be considered, and further studied, as factors potentially influencing not only the mother but the health of the developing offspring."
Elaine Y. Hsiao PhD
Hsiao, Vuong and colleagues reported in 2019 that serotonin and drugs that target serotonin, i.e. antidepressants, can have a major effect on gut microbiota. In 2018, Hsiao and her team established a causal link between seizure susceptibility and gut microbiota, identifying specific gut bacteria that play an essential role in the anti-seizure effects of a ketogenic diet.
Abstract
‘Dysbiosis’ of the maternal gut microbiome, in response to challenges such as infection (1), altered diet (2) and stress (3) during pregnancy, has been increasingly associated with abnormalities in brain function and behaviour of the offspring4. However, it is unclear whether the maternal gut microbiome influences neurodevelopment during critical prenatal periods and in the absence of environmental challenges. Here we investigate how depletion and selective reconstitution of the maternal gut microbiome influences fetal neurodevelopment in mice. Embryos from antibiotic-treated and germ-free dams exhibited reduced brain expression of genes related to axonogenesis, deficient thalamocortical axons and impaired outgrowth of thalamic axons in response to cell-extrinsic factors. Gnotobiotic colonization of microbiome-depleted dams with a limited consortium of bacteria prevented abnormalities in fetal brain gene expression and thalamocortical axonogenesis. Metabolomic profiling revealed that the maternal microbiome regulates numerous small molecules in the maternal serum and the brains of fetal offspring. Select microbiota-dependent metabolites promoted axon outgrowth from fetal thalamic explants. Moreover, maternal supplementation with these metabolites abrogated deficiencies in fetal thalamocortical axons. Manipulation of the maternal microbiome and microbial metabolites during pregnancy yielded adult offspring with altered tactile sensitivity in two aversive somatosensory behavioural tasks, but no overt differences in many other sensorimotor behaviours. Together, our findings show that the maternal gut microbiome promotes fetal thalamocortical axonogenesis, probably through signalling by microbially modulated metabolites to neurons in the developing brain.
Authors
Helen E. Vuong, Geoffrey N. Pronovost, Drake W. Williams, Elena J. L. Coley, Emily L. Siegler, Austin Qiu, Maria Kazantsev, Chantel J. Wilson, Tomiko Rendon and Elaine Y. Hsiao.
AcknowledgementsScience
The authors thank members of the Hsiao lab for their critical review of the manuscript; T. Su for RNA sequencing advice; A. Oyler-Yaniv, J. Oyler-Yaniv and R. Wollman for assistance with initial light-sheet image acquisition; A. Rajbhandari and Irina Zhuravka of the UCLA Behavioral Testing Core for behavioural assay training; S. White for sharing ultrasonic vocalization equipment; and A. Collazo of the Caltech Beckman Institute Biological Imaging Facility for assistance with light-sheet image acquisition and analysis. Support for this research was provided by the Packard Fellowship in Science and Engineering and Klingenstein-Simons Award to E.Y.H.; UPLIFT: UCLA Postdocs’ Longitudinal Investment in Faculty Award (# K12 GM106996) and NICHD Pathway to Independence Award (#K99 HD101680) to H.E.V.; the Ruth L. Kirschstein National Research Service Awards (#F31 HD101270 to G.N.P. and #F30 DE025172 to D.W.W.), and the NSF Graduate Research Fellowship to E.J.L.C. E.Y.H. is a New York Stem Cell Foundation – Robertson Investigator. This research was supported in part by the New York Stem Cell Foundation.
The Nature research was supported by funding from the David and Lucile Packard Foundation's Packard Fellowship for Science and Engineering, a Klingenstein-Simons Fellowship Award, a National Science Foundation Graduate Research Fellowship, the National Institutes of Child Health and Human Development, and the New York Stem Cell Foundation.
Return to top of page.
| |
|
Sep 24 2020 Fetal Timeline Maternal Timeline News
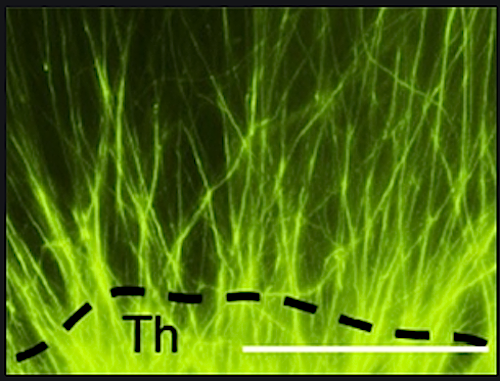 Axon outgrowth [GREEN] from fetal mouse thalamus. Maternal metabolic supplements eliminate deficiencies in fetal axons connecting the thalamus and the cerebral cortex.
|



