|
|
Developmental Biology - Heat & Gene Function
Does Heat Control Gene Function?
Bioengineers are devising a hot new technology to remotely control the positioning and timing of cell functions to build 3-dimensional, artificial, living tissues...
The labs of Kelly Stevens at the UW Medicine Institute of Stem Cell and Regenerative Medicine in Seattle, Washington, and Jordan Miller at Rice University in Houston, Texas, are collaborating to develop bio-printed, organ-like tissues, such as liver and lung constructs.
Steven's lab has the long-term vision of building liver tissues that simulate some of the many, complex functions of that organ. His hope is that artificial tissues could be used to study, for example, how drugs or toxins act on the liver.
However, the liver is prone to damage from infections, medications, poisons and even common intoxicants like alcohol. Liver disease affects more than 500 million people worldwide and accounts for more than 2 million deaths each year. Eventually, researchers would like to use engineered artificial tissues to surgically implant and take over lost function from diseased livers.
As an assistant professor of bioengineering, Stevens is in a department jointly run by the University of Washington (UW) College of Engineering and the UW School of Medicine. She also holds an appointment in the Department of Laboratory Medicine and Pathology at UW Medical School, and knows the intricacies of the liver and the hurdles in artificial organ bioengineering. She has long pondered if cells can be prompted to assume different functional roles as well as spatial positions in a newly generated organ.
Just as employees in a factory have different responsibilities and work locations, so do groups of cells within the liver. Liver cells get job assignments from key genes that, through protein expression profiles, guide them to designated locations to carry out their assignments.
How genes respond to cues which shape a cell's destiny, and how this information transfer occurs, are becoming better understood. However, getting cells to perform information transfer on demand has been elusive. This is especially true for very complexly organized systems.
"The liver performs hundreds of critical functions — so rebuilding liver remains an enormous challenge," explain the scientists, who are not yet able to create small features, such as the distinct metabolic zones found in natural livers.
To build a liver, they must better understand how the organ is assembled and how its physiology is regulated by the genes in cells.
In the Sept. 30 Science Advances, Stevens, and UW bioengineering graduate student Daniel Corbett, along with their collaborators at Rice University, reported their latest bio-printed creation: a thermofluidic technology that can generate patterns that imitate genetic profiles found in human livers.
Through this thermofluidic technology, they designed 3-D printed fluid systems that supply penetrating heat. The energy from these systems - miniature versions of steam-heat radiators in older apartments - allows them to manipulate gene wiring in cells deep inside artificial tissues.
The technology uses thermal patterns to trigger gene expression [function]. The transfer of warmth from printed networks within the tissues activates embedded cell transgenes, that are heat-inducible.
These manipulations could reveal how gene patterns inside different cells drives segregation of tissues and various liver functions. This knowledge could, in the future, not only suggest ideas for sculpting new, working organs from stem cells, but also remotely manage implanted organ tissues to achieve desired therapeutic responses.
The researchers call their technology Heat Exchangers for Actuation of Transcription, or HEAT. Pathways that are activated in their latest project are certain Wnt signaling networks important for regulating development, maintenance and regeneration of body tissues throughout the animal kingdom.
While their reported findings show the potential of HEAT, researchers encountered limitations in space and time in this their first generation, fully controlled heat transfer system.
Design modifications might overcome some present limitations; this approach can also be coupled with other progress in tissue engineering.
The hope is that further advances in solving the enigma of stimulating gene circuits inside 3D tissues will: "Accelerate the development of artificial liver tissues models that could broadly impact basic and translational human biomedicine." Researchers believe such advances will create new avenues for biomanufacturing 3D tissues and organs.
Abstract
Spatial patterns of gene expression in living organisms orchestrate cell decisions in development, homeostasis, and disease. However, most methods for reconstructing gene patterning in 3D cell culture and artificial tissues are restricted by patterning depth and scale. We introduce a depth and scale flexible method to direct volumetric gene expression patterning in 3D artificial tissues, which we call "heat exchangers for actuation of transcription"(HEAT). This approach leverages fluid-based heat transfer from printed networks in the tissues to activate heat-inducible transgenes expressed by embedded cells. We show that gene expression patterning can be tuned both spatially and dynamically by varying channel network architecture, fluid temperature, fluid flow direction, and stimulation timing in a user-defined manner and maintained in vivo. We apply this approach to activate the 3D positional expression of Wnt ligands and Wnt/ß-catenin pathway regulators, which are major regulators of development, homeostasis, regeneration, and cancer throughout the animal kingdom.
Authors
Daniel C. Corbett, Wesley B. Fabyan, Bagrat Grigoryan, Colleen E. O’Connor, Fredrik Johansson, Ivan Batalov, Mary C. Regier, Cole A. DeForest, Jordan S. Miller and Kelly R. Stevens.
Acknowledgements
This research was funded by NIH NHLBI grant DP2HL137188 (K.R.S.), the NIH NIBIB Cardiovascular Training Grant (D.C.C., T32EB001650), the NIH Environmental Pathology and Toxicology Training (W.B.F., T32ES007032), the NSF Graduate Research Fellowship (B.G., 1450681), the Ford Foundation Predoctoral Fellowship (C.E.O.), the Washington Research Foundation postdoctoral fellowship (M.C.R.), the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (J.S.M.), and the Gree Foundation (I.B., K.R.S., and C.A.D.). We thank the large number of open-source and related projects that critically facilitated this work, including Arduino.cc, RepRap.org, UltiMachine.com, Ultimaker.com, Blender.org, Python.org, ImageMagick.org, Git, NIH ImageJ, Fiji.sc, and the NIH 3D Print Exchange. We thank J. Jang, H. Reincke, P. Grosjean, Y. Zheng, B. Ratner, and A. Emery for helpful discussions. We thank D. Hailey from the UW Garvey Imaging Core for assistance with imaging, S. Halabiya, and C. Cordray for assistance with subcloning, and N. Paragas for assistance with the IVIS Imaging System. Author contributions: D.C.C. and K.R.S conceived and initiated the project. All authors contributed to experimental design, experimental planning, experimental execution, data analysis, and to the writing of the manuscript. Competing interests: J.S.M. and B.G. are co-founders of and hold an equity stake in Volumetric Inc., which is a startup company. D.C.C., K.R.S., B.G., and J.S.M. are listed as co-inventors in a pending U.S. Patent application 62/756,106. Remaining co-authors report that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Data and hydrogel design files for 3D printing are available at Zenodo (https://zenodo.org/record/4014310#.X1Fxz8hKhPY).
Computer code generated to analyze data sets is available in Zenodo. Data, hydrogel design files, and computer code are available in Zenodo. Additional data related to this paper may be requested from the authors. Computer code generated to analyze datasets is available in Zenodo. Data, hydrogel design files, and computer code are available in Zenodo.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial license, which permits use, distribution, and reproduction in any medium, so long as the resultant use is not for commercial advantage and provided the original work is properly cited.
https://creativecommons.org/licenses/by-nc/4.0/
Return to top of page.
|
|
Oct 12 2020 Fetal Timeline Maternal Timeline News
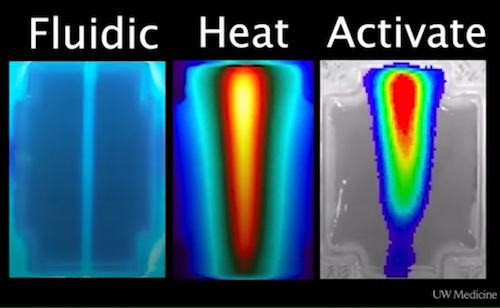 Bioengineers and pathologists are engineering heat to direct cell function in hopes of creating artificial tissues. LEFT Lab engineered Fluidic Device; MIDDLE Heat activation of device; RIGHT: Cells adapting to heat levels. CREDIT
University of Washington. VIDEO
| |



