|
|
Developmental Biology - Brain Development
New Source of Prenatal Inflammation?
Fragile brain tissue extensions protect the developing brain - but may also pass on inflammation from mother to fetus...
Floating in fluid deep within our brain are delicate, small and not well understood brain tissues known as the Choroid Plexus (ChP). Two new studies are now revealing that these are miniature organs and a hotbed of immune system activity. Activity that may protect the fetal brain from infections - but might also contribute to neurodevelopmental disorders such as autism.
"There is a correlation between maternal illness during pregnancy and autism, and we want to investigate and determine how this is happening. A very challenging process to study in the lab."
Maria Lehtinen PhD, Neurobiologist, Boston Children's Hospital, leader of both studies.
The Lehtinen laboratory, part of Boston Children's Department of Pathology, is one of a few labs in the world studying the choroid plexus. Anchored in each of the four ventricles of the brain, the Choroid Plexus produces cerebrospinal fluid (CSF) that bathes the brain and spinal cord. Lehtinen's research has also shown that the choroid plexus influences brain development. It secretes instructive cues into the CSF, while providing a protective brain barrier in early life, preventing unwanted cells or molecules in blood from circulating freely through the growing brain.
But what happens when this barrier is challenged? The lab's newest work offers an unprecedented real-time view of the choroid plexus in a mouse model. The technique also provides a glimpse of how disturbances in the mother's immune system in pregnancy can disrupt a developing brain. The research reveals that the choroid plexus can become a conduit for inflammation resulting from maternal infection, environmental stress and other factors.
A Window On the Choroid Plexus
Laying so deeply in the brain, the choroid plexus is normally very difficult to view. In their first study, published 5 September 2018 in Neuron, the researchers built special imaging tools to capture cellular activity in and around the choroid plexus of adult mice.
Led by Frederick Shipley, Neil Dani, Maria Lehtinen and Mark Andermann all at Beth Israel Deaconness Medical Center, the team carefully removed part of a mouse skull and inserted a "window" of clear plexiglass — to create a "skylight" into its living brain. Using live two-photon imaging to provide three-dimensional views of deep tissues, the team observed the choroid plexus in real time. They were able to track the movements of immune cells, monitored changes in calcium (a proxy for cell activity), and measure cell secretions within the choroid plexus.
Choroid Plexus/Inflammation/& Developing Brain
The second study, published 9 October 2020, in Developmental Cell and led by Jin Cui PhD, applied similar techniques to observe the effects of maternal inflammation on the brains of embryonic mice. To mimic maternal inflammation, the team introduced a molecular messenger known as a cytokine to artificially trigger an inflammatory immune response. Embryos were then collected and carefully placed in an imaging chamber where two-photon imaging of their brains was conductedof through tiny brain "skylights."
The mock maternal inflammation was enough to draw immune cells known as macrophages to an embryo's choroid plexus. Photon imaging showed choroid plexus cells conducting active "surveillance" even in early embryonic stages.
Lehtinen: "The embryonic brain is very small, so it's hard to get good resolution, but we could see macrophages moving and extending as if sampling their environment. This has never been captured before."
Markers of Future Autism?
The team also found an increase in inflammatory signals, particularly CCL2, within the embryonic CSF, evidence these signals were produced by immune cells at the choroid plexus. "Many of these markers, including CCL2, are also upregulated in autism patients," noted Cui.
Further experiments showed that CCL2, on its own, was sufficient to recruit and activate immune cells at the choroid plexus. Looking at tissue specimens, the team saw evidence that macrophages had breached the choroid plexus barrier, crossing into the CSF from specific "hotspots" at the tips of the choroid plexus.
"We have added evidence that the inflammatory response perturbs the development of the brain," explained Cui. "Previous studies from others have shown that maternal inflammation causes brain malformations in mouse models very early in life, and similar malformations can be seen in some autism patients."
A Treatment to Protect the Brain?
In some of their experiments, researchers observed patches of brain disorganization after mouse pups were born. But, much more work is needed to connect maternal inflammation to the choroid plexus and disorders like autism. Even more work will be needed to translate newly gained knowledge into treatment.
"Our goal will be to see if preventing breach of the choroid plexus barrier — could slow or prevent progression of disease in the brain. That will involve collaborating with many different groups in multiple fields, as well as advances in imaging technology currently underway."
Maria K. Lehtinen PhD
Abstract
Highlights
• Embryonic ChP mounts inflammatory response to maternal infection
• Embryonic CSF contains key biomarkers of neurodevelopmental disorders
• Two-photon imaging reveals macrophage motility and mobility at embryonic ChP
• CCL2 weakens ChP barrier and recruits immune cells through ChP barrier hotspots
Summary
The choroid plexus (ChP) regulates brain development by secreting instructive cues and providing a protective brain barrier. Here, we show that polyI:C-mediated maternal immune activation leads to an inflammatory response in the developing embryonic mouse brain that manifests as pro-inflammatory cerebrospinal fluid (CSF) and accumulation of ChP macrophages. Elevation of CSF-CCL2 was sufficient to drive ChP immune cell recruitment, activation, and proliferation. In addition, ChP macrophages abandoned their regular tiling pattern and relocated to the ChP-free margin where they breached the weakened epithelial barrier. We further found that these immune cells entered from the ChP into the brain via anatomically specialized “hotspots” at the distal tips of ChP villi. In vivo two-photon imaging demonstrated that surveillance behaviors in ChP macrophages had already emerged at this early stage of embryogenesis. Thus, the embryonic ChP forms a functional brain barrier that can mount an inflammatory response to external insults.
Authors
JinCui, Frederick B.Shipley, Morgan L.Shannon, Osama Alturkistani, Neil Dani, Mya D. Webb, Arthur U. Sugden, Mark L. Andermann, Maria K. Lehtinen.
Acknowledgements
These studies' many supporters include the National Institutes of Health, a William Randolph Hearst Fellowship, the National Science Foundation, the New York Stem Cell Foundation, the Pediatric Hydrocephalus Foundation, the Tommy Fuss Center, the Simons Foundation, and the Harvard Brain Science Initiative.
Return to top of page.
|
|
Oct 13 2020 Fetal Timeline Maternal Timeline News
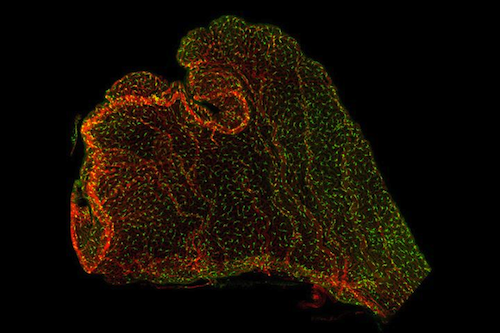 The choroid plexus are tiny brain tissue extensions within each of the four ventricles of the brain. CREDIT Lehtinen Lab, Children’s Hospital, Boston, MA.
| |



