|
|
Developmental Biology - DNA, Proteins and Phase Separation
DNA Interactions Helped Shape All Life On Earth
Simple DNA-peptide interactions create a surprising diversity of phase behaviours, suggesting these polymers' interactions helped create today's complex biological structures...
Deoxyribonucleic acid (DNA)-protein interactions are extremely important in biology. Every human cell contains about 2 meters (approx. 6 feet) worth of DNA, packaged into an infinitesimally small cell by wrapping the DNA around proteins. The information in DNA allows a cell to copy itself. So, how DNA and proteins interact is of extreme interest to scientists trying to understand how all biological life is organised.
New research published in the journal of the ACS (American Chemical Society) by scientists at the Earth-Life Science Institute (ELSI), Tokyo Institute of Technology along with the Institut Pierre-Gilles de Gennes, ESPCI Paris, Université PSL suggests that interactions between DNA and proteins reflect deep-seated properties in each to form higher-ordered structures which allow for such extreme adaptations such as packaging DNA into a cell.
Modern cells are principally made up of a few classes of large molecules. DNA gets the lion's share of our attention, as it is houses the information cells use to rebuild themselves generation after generation.
Information-rich DNA is normally presented as a double-stranded caduceus of two polymers wrapped around each other. Much of the information DNA contains is obscured to the external environment as the information-bearing parts of the molecule are locked into their complementary strand.
When DNA is copied into ribonucleic acid (RNA), both strands are pulled apart to allow their more complex surfaces to interact — which also enables it to be copied into single-stranded RNA polymers. These RNA polymers are then read via biological processes into proteins. Proteins themselves are polymers. They are a variety of amino acids with extremely complicated surface properties. Thus, DNA and RNA are somewhat predictable chemically in behaviour as polymers — but proteins are not.
Polymeric molecules, those that are made up of repeated types of subunits, display complex behaviours when mixed with other chemicals — especially if dissolved in a solvent like water. Chemists have developed a complex set of terms for how compounds behave when they are mixed.
For example, the proteins in cow's milk are considered a colloidal suspension in water — they become a homogeneous, noncrystalline suspension which does not settle and cannot be separated by any physical means.
However, when lemon juice is added to milk, the suspended cow milk proteins reorganise themselves and produce the very visible self-organisation of curds, which represent a new transition phase.
Other types of polymer phenomenon have been discovered over the years, for example, liquid crystals (LC). LCs are formed when molecules have an elongated shape and have the tendency to aggregate one on top of the other. This results in a material with the properties of a crystal and a liquid. Liquid crystals have a certain degree of order like a solid (the parallel orientation of molecules) but still retain fluidity (molecules that can easily slip by each other).
We all experience liquid crystals in the various TV, computer and wrist watch screens we interact with every day. "LCDs" or Liquid Crystals Displays, use these same properties.
In their research, Fraccia and Jia, show that double-stranded DNA and peptides together generate many different LC phases in a very peculiar way. LC phases actually form in membraneless droplets, called coacervates, where DNA and peptides spontaneously co-assemble and become ordered.
This process brings DNA and peptides together in a very high concentrate. Comparable to that of a cell nucleus — or 100 to 1,000 times denser then concentrations likely achieved on early Earth.
Such a spontaneous behaviour could have — in principle — supported the formation of the first cell-like structures on early Earth. Taking advantage of order plus fluidity, to gain stability and functionality, and favor the growth and continuous evolution of primitive biomolecules.
The cut-off between each higher-order cell-like property is not clear cut. When molecules interact, they often "self-organise." Think of the process of adding sand to a sandpile: as you sprinkle more and more sand onto a sand pile, it tends to form a "low energy" final state - simply a pile. Though the addition of sand grains may cause new 'structures' to form in the local spot where they fall, at some point adding one more grain causes a landslide. Now the pile will return to the conical shape of the original pile.
Though we all benefit from the existence of these phenomena, Jia and Fraccia argue we may be missing other important implications of this type of self-organisation. Combining these self-organising effects may be relevant to many levels of biology. Even important in biomolecular transitions in cell physiology as well as disease. In particular, the authors discovered various liquid crystalline structures could be continuously accessed simply by changing environmental conditions. Such as simply changing salinity or environmental temperature. Given the numerous unexplored conditions, this work suggests many more self-organised LC mesophases with potential biological function could be discovered in the near future.
This new understanding of bio-polymeric self-organisation may also be important to understanding how life self-organised into becomming alive in the first place. Understanding how primitive collections of molecules could have structured themselves into collectively behaving as aggregates, is a significant avenue for future research.
"When the general public hears about liquid crystals, they might only think of TV screens and engineering applications. Very few will immediately think of basic science. Most researchers won't even make the connection between LCs and the origins of life. We hope this work will help increase public and scientific understanding of LCs in the context of the origins of life."
Tony Z. Jia PhD, Earth-Life Science Institute, Tokyo Institute of Technology, Tokyo, Japan; Blue Marble Space Institute of Science, Seattle, Washington, USA.
Finally, this work may be relevant to disease. Recent discoveries regarding diseases including Alzheimer's, Parkinson's, Huntington's Disease, and ALS (Lou Gehrig's Disease) have pointed to intracellular phase transitions and separations leading to membraneless droplets as a potential cause.
Researchers note that although their work was heavily impacted by the pandemic, they did their best to keep working under the global shutdowns and travel restrictions.
Abstract
Phase separation of nucleic acids and proteins is a ubiquitous phenomenon regulating subcellular compartment structure and function. While complex coacervation of flexible single-stranded nucleic acids is broadly investigated, coacervation of double-stranded DNA (dsDNA) is less studied because of its propensity to generate solid precipitates. Here, we reverse this perspective by showing that short dsDNA and poly-l-lysine coacervates can escape precipitation while displaying a surprisingly complex phase diagram, including the full set of liquid crystal (LC) mesophases observed to date in bulk dsDNA. Short dsDNA supramolecular aggregation and packing in the dense coacervate phase are the main parameters regulating the global LC-coacervate phase behavior. LC-coacervate structure was characterized upon variations in temperature and monovalent salt, DNA, and peptide concentrations, which allow continuous reversible transitions between all accessible phases. A deeper understanding of LC-coacervates can gain insights to decipher structures and phase transition mechanisms within biomolecular condensates, to design stimuli-responsive multiphase synthetic compartments with different degrees of order and to exploit self-assembly driven cooperative prebiotic evolution of nucleic acids and peptides.
Authors
Tommaso P. Fraccia and Tony Z. Jia.
Acknowledgements
Tommaso P. Fraccia1,†,* & Tony Z. Jia2,3,†1Institut Pierre-Gilles de Gennes, Chimie Biologie et Innovation, ESPCI Paris, PSL University, CNRS, 75005 Paris, France2Earth-Life Science Institute, Tokyo Institute of Technology, 2-12-1-IE-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan3Blue Marble Space Institute of Science, 1001 4th Ave., Suite 3201, Seattle, Washington 98154 USA†These authors contributed equally.
Return to top of page.
|
|
Oct 15 2020 Fetal Timeline Maternal Timeline News
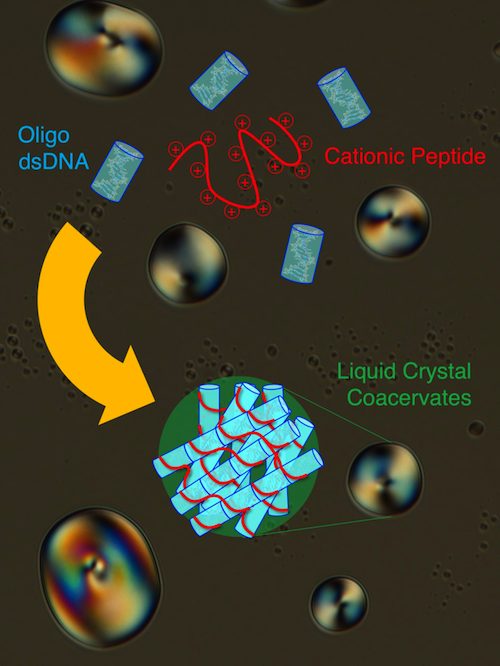 This graph shows how short double-stranded DNA of Oligo dsDNA assemble into end-to-end stacks (BLUE) and merge with a Cationic Peptide (poly-L-lysine) (RED) to form rigid bundles of Liquid Crystal Coacervate in droplets (GREEN). CREDIT Tommaso P Fraccia
| |



