|
|
Developmental Biology - Genetic Disorders
A Protein That Stops Deadly COVID-19 Reactions
Blocking protein factor D may stop COVID-19 infection, prevent severe organ damage...
While the world waits eagerly for a safe and effective vaccine to prevent infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) the virus behind COVID-19 pandemic, researchers focus on how SARS-CoV-2 attacks the body in order to stop its devastating impact. One possibility is blocking a protein that turns the immune system against healthy cells. Johns Hopkins Medicine researchers identified it recently.
Based on their findings, researchers believe inhibiting the protein: Factor D will curtail the potentially deadly inflammatory reactions that many patients have to the virus.
Making the discovery even more exciting is that there may already be drugs in development and testing for other diseases that can do the required blocking. The work is published in the Sept. 2, 2020, issue of the journal Blood.
Scientists already know that spike proteins on the surface of the SARS-CoV-2 virus (making the pathogen look like the spiny ball from a medieval mace) are the means by which it attaches to cells targeted for infection. The spikes first grab hold of heparan sulfate, a large, complex sugar molecule found on the surface of cells in the lungs, blood vessels and the smooth muscle making up most organs. Facilitated by its initial binding with heparan sulfate, SARS-CoV-2 then uses another cell-surface component, the protein known as angiotensin-converting enzyme 2 (ACE2), as its doorway into the attacked cell.
The Johns Hopkins Medicine team discovered that when SARS-CoV-2 ties up heparan sulfate, it prevents factor H from using the sugar molecule to bind with cells. Factor H's normal function is to regulate the chemical signals that trigger inflammation and keep the immune system from harming healthy cells. Without this protection, cells in the lungs, heart, kidneys and other organs can be destroyed by the defense mechanism nature intended to have safeguard them.
"Previous research suggested that along with tying up heparan sulfate, SARS-CoV-2 activates a cascading series of biological reactions. What we call the Alternative Pathway of Complement (APC) - can lead to inflammation and cell destruction of healthy organs, if misdirected by the immune system. The goal of our study was to discover how the virus activates this pathway and to find a way to inhibit it before the damage occurs."
Robert Brodsky MD, Director, Hematology Division, Johns Hopkins University School of Medicine and senior author.
The APC is one of three chain reaction processes involving splitting and recombining more than 20 different proteins - known as complement proteins - that usually get activated when bacteria or viruses invade the body. The end product of this complement cascade, a structure called membrane attack complex (MAC), forms on the surface of the invader and causes its destruction, either by creating holes in bacterial membranes or disrupting a virus' outer envelope. However, MACs also can arise on the membranes of healthy cells. Fortunately, humans have a number of complement proteins, including factor H, that regulate the APC, keep it in check and therefore, protect normal cells from damage by MACs.
In a series of experiments, Brodsky and his colleagues used normal human blood serum and three subunits of the SARS-CoV-2 spike protein to discover exactly how the virus activates the APC, hijacks the immune system and endangers normal cells. They discovered that two of the subunits, called S1 and S2, are the ones that bind the virus to heparan sulfate - setting off the APC cascade and blocking factor H from connecting with the sugar - and in turn, disabling the complement regulation by which factor H deters a misdirected immune response.
In turn, researchers believe the resulting immune system response to chemicals released by the lysing of killed cells could be responsible for the organ damage and failures seen in severe cases of COVID-19.
Most notably, Brodsky says, the research team found by blocking another complement protein, known as factor D, which works immediately upstream in the pathway from factor H, they were able to stop the destructive chain of events triggered by SARS-CoV-2.
"When we added a small molecule that inhibits the function of factor D, the APC wasn't activated by the virus spike proteins," Brodsky says. "We believe that when the SARS-CoV-2 spike proteins bind to heparan sulfate, it triggers an increase in the complement mediated killing of normal cells because factor H, a key regulator of the APC, can't do its job."
To better understand what happens, Brodsky says, think of the APC like a car in motion.
"If the brakes are disabled, the gas pedal can be floored without restraint, very likely leading to a crash and destruction," he explains. "The viral spike proteins disable the biological brakes, factor H, enabling the gas pedal, factor D, to accelerate the immune system and cause cell, tissue and organ devastation. Inhibit factor D, and the brakes can be reapplied and the immune system reset."
Brodsky adds that cell death and organ damage from a misdirected APC associated with factor H suppression is already known to occur in several complement-related human diseases, including age-related macular degeneration, a leading cause of vision loss for people age 50 and older; and atypical hemolytic uremic syndrome (aHUS), a rare disease that causes clots to block blood flow to the kidneys.
Brodsky and his colleagues hope that their work will encourage more study into the potential use against COVID-19 of complement-inhibiting drugs already in the pipeline for other diseases.
"There are a number of these drugs that will be FDA-approved and in clinical practice within the next two years. Perhaps one or more of these could be teamed with vaccines to help control the spread of COVID-19 and avoid future viral pandemics."
Robert A. Brodsky .
Abstract
Key Points
• SARS-CoV-2 spike proteins bind heparan sulfate and activate the alternative complement pathway on cell surfaces.
• Factor D inhibitor (ACH-145951) blocks the complement activation induced by SARS-CoV-2 spike proteins.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a highly contagious respiratory virus that can lead to venous/arterial thrombosis, stroke, renal failure, myocardial infarction, thrombocytopenia, and other end-organ damage. Animal models demonstrating end-organ protection in C3 deficient mice and evidence of complement activation in humans have led to the hypothesis that SARS-CoV-2 triggers complement-mediated endothelial damage, but the mechanism is unclear. Here, we demonstrate that SARS-CoV-2 spike protein (subunit 1 and 2), but not N protein, directly activates the alternative pathway of complement (APC). Complement dependent killing using the modified Ham test is blocked by either C5 or factor D inhibition. C3 fragments and C5b-9 are deposited on TF1PIGAnull target cells, and complement factor Bb is increased in the supernatant from spike protein treated cells. C5 inhibition prevents the accumulation of C5b-9 on cells, but not C3c; however, factor D inhibition prevents both C3c and C5b-9 accumulation. Addition of factor H mitigates the complement attack. In conclusion, SARS-CoV-2 spike proteins convert non-activator surfaces to activator surfaces by preventing the inactivation of the cell surface APC convertase. APC activation may explain many of the clinical manifestations (microangiopathy, thrombocytopenia, renal injury, and thrombophilia) of COVID-19 that are also observed in other complement-driven diseases such as atypical hemolytic uremic syndrome and catastrophic antiphospholipid antibody syndrome. C5 inhibition prevents accumulation of C5b-9 in vitro but does not prevent upstream complement activation in response to SARS-CoV-2 spike proteins.
Authors
Vincenzo Galasso, Vincent Pons, Paola Profeta, Michael Becher, Sylvain Brouard and Martial Foucault.
Acknowledgements
This study was supported by National Heart, Lung and Blood Institute grant R01 HL133113.
Disclaimer: Johns Hopkins Medicine researchers are working tirelessly to find ways to better understand and eventually eliminate COVID-19 and the virus that causes it. Discoveries like this one, especially those related to clinical therapies and drug regimens, are still early in concept and small in sample size. This will require rigorous research, testing and peer review, all of which take time, before solid conclusions for clinical care and disease prevention can be made.
Return to top of page.
| |
|
Oct 22 2020 Fetal Timeline Maternal Timeline News
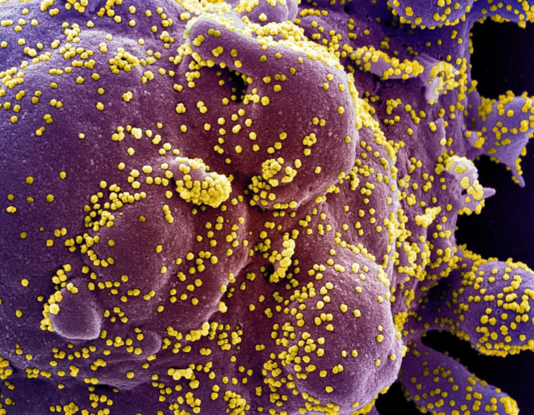 Electron micrograph of cell (PURPLE) heavily infected with SARS-CoV-2 virus particles (YELLOW). Johns Hopkins Medicine shows blocking one specific protein may prevent SARS-CoV-2 infection from misdirecting immune system attacks on healthy cells. CREDIT National Institute of Allergy and Infectious Diseases/ NIH.
|



