|
|
Developmental Biology - Alzheimer's
New Blood Test for Alzheimer's
A new blood test for Alzheimer's disease has been developed under the leadership of researchers at the University of Gothenburg, Sweden...
The testing method is based on measuring a specific variant of tau protein in ordinary blood samples, which makes the test relatively simple and cheap to perform.
The research behind the test was headed by Kaj Blennow, Professor of Clinical Neurochemistry, and Henrik Zetterberg, Professor of Neurochemistry, at Sahlgrenska Academy, University of Gothenburg. The results are published in an article in The Lancet Neurology.
Alzheimer's disease is characterized by two pathological changes in the tissue of the nervous system:
One: the formation of extracellular clumps of protein called beta-amyloid.
The other: neurofibrils, made up of tau protein, stuck together in tiny, neurofibrillary tangles - a biochemical process known as phosphorylation.
The newly developed method is based on measurement of phosphorylated tau — specifically, the P-tau181 variant — in ordinary blood samples, performed with an ultrasensitive method known as Single Molecule Array (Simoa). Simoa can measure considerably lower levels of protein biomarkers than other analytical methods.
P-tau181 has long been measurable through testing of cerebrospinal fluid, in which it is found at a considerably higher level than in blood samples. For the past few years, it has also been possible to demonstrate neurofibrils by using the advanced positron emission tomography (PET) medical imaging technique. Tests of cerebrospinal fluid are, however, difficult to perform in primary care, and the high costs of PET scans restrict their use.
Being able to establish tau pathology through ordinary blood tests is therefore, highly valuable.
The results now published show that the level of P-tau181 is greatly elevated in Alzheimer's, including at its early stage, known as mild cognitive impairment. However, this raised level was found only in patients who also had amyloid plaques, as revealed by the PET camera.
The level of specific P-tau181 in blood plasma also proved to correspond very closely with the level of tau tangles in the brain registered with the PET-technique. The blood test also identified people early on in the course of the disease who had plaques, but in whom the PET technique discerned no increased tau levels.
The blood test showed a very good capacity to distinguish Alzheimer's from other brain diseases, such as frontotemporal dementia and Parkinson's disease, where the blood level of P-tau181 was entirely normal.
The blood test developed at the University of Gothenburg produces results similar to those from the blood test that was developed at the pharmaceutical company Eli Lilly (USA). Results from the latter were recently published in Nature Medicine, with Kaj Blennow and Henrik Zetterberg as coauthors.
"We believe that, in the future, one very important use of our blood test will be for screening in primary care. We demonstrated this in one of the studies forming part of our article, in which we looked at patients in primary care with concerns about their failing memory."
Kaj Blennow MD, Professor, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden.
"We also think the level of P-tau181 in blood plasma may be a very important marker to show and monitor the efficacy of the new drugs against Alzheimer's that are currently being developed."
Henrik Zetterberg MD, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden; Department of Neurodegenerative Disease, Institute of Neurology and UK Dementia Research Institute, University College London, UK.
Summary
Background
CSF and PET biomarkers of amyloid ß and tau accurately detect Alzheimer's disease pathology, but the invasiveness, high cost, and poor availability of these detection methods restrict their widespread use as clinical diagnostic tools. CSF tau phosphorylated at threonine 181 (p-tau181) is a highly specific biomarker for Alzheimer's disease pathology. We aimed to assess whether blood p-tau181 could be used as a biomarker for Alzheimer's disease and for prediction of cognitive decline and hippocampal atrophy.
Methods
We developed and validated an ultrasensitive blood immunoassay for p-tau181. Assay performance was evaluated in four clinic-based prospective cohorts. The discovery cohort comprised patients with Alzheimer's disease and age-matched controls. Two validation cohorts (TRIAD and BioFINDER-2) included cognitively unimpaired older adults (mean age 63–69 years), participants with mild cognitive impairment (MCI), Alzheimer's disease, and frontotemporal dementia. In addition, TRIAD included healthy young adults (mean age 23 years) and BioFINDER-2 included patients with other neurodegenerative disorders. The primary care cohort, which recruited participants in Montreal, Canada, comprised control participants from the community without a diagnosis of a neurological condition and patients referred from primary care physicians of the Canadian National Health Service for specialist care. Concentrations of plasma p-tau181 were compared with established CSF and PET biomarkers and longitudinal measurements using Spearman correlation, area under the curve (AUC), and linear regression analyses.
Findings
We studied 37 individuals in the discovery cohort, 226 in the first validation cohort (TRIAD), 763 in the second validation cohort (BioFINDER-2), and 105 in the primary care cohort (n=1131 individuals). In all cohorts, plasma p-tau181 showed gradual increases along the Alzheimer's disease continuum, from the lowest concentrations in amyloid ß-negative young adults and cognitively unimpaired older adults, through higher concentrations in the amyloid ß-positive cognitively unimpaired older adults and MCI groups, to the highest concentrations in the amyloid ß-positive MCI and Alzheimer's disease groups (p<0·001, Alzheimer's disease vs all other groups). Plasma p-tau181 distinguished Alzheimer's disease dementia from amyloid ß-negative young adults (AUC=99·40%) and cognitively unimpaired older adults (AUC=90·21–98·24% across cohorts), as well as other neurodegenerative disorders, including frontotemporal dementia (AUC=82·76–100% across cohorts), vascular dementia (AUC=92·13%), progressive supranuclear palsy or corticobasal syndrome (AUC=88·47%), and Parkinson's disease or multiple systems atrophy (AUC=81·90%). Plasma p-tau181 was associated with PET-measured cerebral tau (AUC=83·08–93·11% across cohorts) and amyloid ß (AUC=76·14–88·09% across cohorts) pathologies, and 1-year cognitive decline (p=0·0015) and hippocampal atrophy (p=0·015). In the primary care cohort, plasma p-tau181 discriminated Alzheimer's disease from young adults (AUC=100%) and cognitively unimpaired older adults (AUC=84·44%), but not from MCI (AUC=55·00%).
Interpretation
Blood p-tau181 can predict tau and amyloid ß pathologies, differentiate Alzheimer's disease from other neurodegenerative disorders, and identify Alzheimer's disease across the clinical continuum. Blood p-tau181 could be used as a simple, accessible, and scalable test for screening and diagnosis of Alzheimer's disease.
Funding
Alzheimer Drug Discovery Foundation, European Research Council, Swedish Research Council, Swedish Alzheimer Foundation, Swedish Dementia Foundation, Alzheimer Society Research Program.
Authors
Thomas K Karikari PhD, Tharick A Pascoal MD, Nicholas J Ashton PhD, Shorena Janelidze PhD, Andréa Lessa Benedet MSc, Juan Lantero Rodriguez MSc, Mira Chamoun PhD, Melissa Savard MSc, Min Su Kang BSc, Joseph Therriault BSc, Michael Schöll PhD, Gassan Massarweh PhD, Jean-Paul Soucy MD, Kina Höglund PhD, Gunnar Brinkmalm PhD, Niklas Mattsson MD, Sebastian Palmqvist MD, Prof Serge Gauthier MD, Erik Stomrud PhD, Prof Henrik Zetterberg MD, Prof Oskar Hansson PhD, Prof Pedro Rosa-Neto MD, Prof Kaj Blennow MD.
Return to top of page.
| |
|
Nov 4 2020 Fetal Timeline Maternal Timeline News
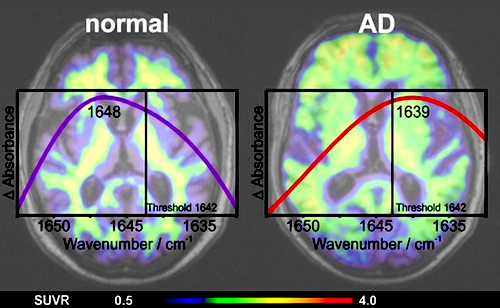 Amyloid-ß distribution in blood plasma by an immuno-IR-sensor, correlates with PET scanning and CSF markers in Alzheimer's disease (AD) patients - at much less cost. CREDIT EMBO.
|



