|
|
Developmental Biology - Alzheimer's
Alzheimer's Traced to Mutation in Common Enzyme
Mutation of common brain enzyme makes protein sticky...
Alzheimer's disease is a life-changing, debilitating condition affecting tens of millions of people worldwide. According to the World Health Organization, it causes senile dementia and is expected to double every 20 years - if unchecked.
Now, researchers from Tokyo Metropolitan University have discovered a new mechanism by which clumps of tau protein are created in the brain, killing brain cells and causing Alzheimer's. This research appears in JBC the Journal of Biological Chemistry.
A specific mutation in the enzyme MARK4 changes the properties of tau, usually an important part of the skeletal structure of cells, making tau more likely to aggregate as well as more insoluble. Understanding this cellular interaction may lead to breakthrough treatments for such cellular errors.
Alzheimer's is said to be caused by build-up of tangled clumps of tau protein found in brain cells.
Sticky tau protein aggregates cause neurons to die and lead to impaired memory and motor functions. It isn't clear, yet, how and why tau builds up in the brain cells of Alzheimer's patients.
Understanding the cause and mechanism behind this unwanted clumping would open up new treatments and ways to prevent the disease.
A team led by Associate Professor Kanae Ando of Tokyo Metropolitan University has been exploring the role played by the MARK4 (Microtubule Affinity Regulating Kinase 4) enzyme in creating an Alzheimer's disease response. When everything is working normally, tau protein is an important part of cell structure in the cytoskeleton. Tau supports cytoskeleton arms - or microtubules - which constantly help cells maintain their shape and internal organization during cell division. MARK4 actually helps tau detach from the arms of this cytoskeleton structure as needed.
Problems begin when a mutation happens in the MARK4 gene - altering its blueprint instructions.
Previous research associated this error with an increased risk of Alzheimer's, but it was not understood how this occurs. So researchers artificially introduced mutations into transgenic drosophila fruit flies to produce human tau, in order to study how these proteins changed in a living organism.
They discovered that a mutant form of MARK4 makes changes to the tau protein, creating a pathological form of tau. Not only does this "bad" tau have an excess of certain chemical groups that cause it to misfold, it aggregates much more easily and is no longer soluble in detergents. This made it easier for tau to form into tangled clumps that cause neurons to degenerate.
MARK4 is also found to cause a wide range of other diseases involved in aggregation and buildup of other proteins. The team's insights into tau protein buildup may lead to new treatments and measures which prevent even wider varieties of neurodegenerative conditions.
Abstract
Accumulation of the microtubule-associated protein tau is associated with Alzheimer’s disease (AD). In AD brain, tau is abnormally phosphorylated at many sites, and phosphorylation at Ser262 and Ser356 play critical roles in tau accumulation and toxicity. Microtubule-affinity regulating kinase 4 (MARK4) phosphorylates tau at those sites, and a double de novo mutation in the linker region of MARK4, DeltaG316E317D, is associated with an elevated risk of AD. However, it remains unclear how this mutation affects phosphorylation, aggregation, and accumulation of tau and tau-induced neurodegeneration. Here, we report that MARK4 DeltaG316E317D increases the abundance of highly phosphorylated, insoluble tau species, and exacerbates neurodegeneration via Ser262/356-dependent and -independent mechanisms. Using transgenic Drosophila expressing human MARK4 (MARK4wt) or a mutant version of MARK4 (MARK4 DeltaG316E317D), we found that co-expression of MARK4wt and MARK4 DeltaG316E317D increased total tau levels and enhanced tau-induced neurodegeneration, and that MARK4 DeltaG316E317D had more potent effects than MARK4wt. Interestingly, the in vitro kinase activities of MARK4wt and MARK4 DeltaG316E317D were similar. When tau phosphorylation at Ser262 and Ser356 was blocked by alanine substitutions, MARK4wt did not promote tau accumulation or exacerbate neurodegeneration, while co-expression of MARK4 DeltaG316E317D did. Both MARK4wt and MARK4 DeltaG316E317D increased the levels of oligomeric forms of tau; however, only MARK4 DeltaG316E317D further increased the detergent insolubility of tau in vivo. Together, these findings suggest that MARK4DeltaG316E317D increases tau levels and exacerbates tau toxicity via a novel gain-of-function mechanism, and that modification in this region of MARK4 may impact disease pathogenesis.
Authors
Toshiya Oba, Taro Saito, Akiko Asada, Sawako Shimizu, Koichi M Iijima and Kanae Ando.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control) [JSPS KAKENHI Grant number 17H05703], a research award from the Hoan-sha Foundation, the Takeda Science Foundation, a research award from the Japan Foundation for Aging and Health, a Grant-in-Aid for Scientific Research on Challenging Research (Exploratory) [JSPS KAKENHI Grant number 19K21593], and Research Funding for Longevity Science 19-7 from the National Center for Geriatrics and Gerontology, Japan.
Return to top of page.
| |
|
Nov 5 2020 Fetal Timeline Maternal Timeline News
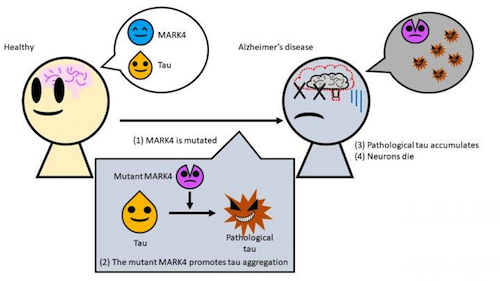 The mutant MARK4 enzyme creates a form of TAU protein that easily accumulates in brain cells, causing neurons to die. CREDIT Tokyo Metropolitan University.
|



