|
|
Developmental Biology - Stem Cells
Why Stem Cells Can Endlessly Divide
The centromere, a structure connecting each pair of chromosomes found in our body, is smaller and weaker in stem cells — allowing those cells to divide almost endlessly into new cell types...
Stem cells are one of the most promising tools in regenerative medicine. They can recreate every cell type in our body — giving medicine the potential to replace tissue cells lossed to damage or disease. With this flexibility, they are similar to embryonic cells which generate every cell type needed in the rapidly growing fetus.
But, stem cells can more easily be generated in the laboratory, from skin cells for example. Reprograming the function (or 'expression') of a stem cell from a previously spcialized or differentiated cell type, earned the Nobel Prize in Physiology or Medicine in 2012.
Despite this incredible potential to regenerate, little is known about the mechanisms that govern stem cell division. This is important to understand as they, and all cells, have a natural tendency to accumulate errors during separation of their chromosomes. Stem cells can duplicate almost indefinitely and one of the elements needed for a successful cell division (mitosis) is the centromere.
The centromere is the binding site of every chromosome protein complex. It is where, after being duplicated and condensed, genes are ensured to be equally distributed between the two new daughter-cells just created.
Driven to understand stem cell chromosome segregation, Raquel Oliveira and Lars Jansen led a team of Instituto Gulbenkian de Ciência (IGC) researchers, in a fundamental project on centromeres and their protein complexes. The research appears in the journal Open Biology a publication of the Royal Society, the United Kingdom’s National Science Academy.
"A precise definition of the composition and size of the centromere in stem cells was made, revealing that stem cell chromosomes have weaker centromeres when compared to the ones of differentiated cells. Moreover, these structures become weaker as a consequence of acquiring the identity of 'stem cell'."
Inez Milagre, Instituto Gulbenkian de Ciência (IGC), Rua da Quinta Grande, Portugal and first author of the study.
"This 'weakness' in a structure of such importance for the correct distribution of chromosomes between daughter-cells might explain why stem cells make more mistakes when they divide."
Lars Jansen, Principal Investigator, IGC, Oeiras, Portugal; Department of Biochemistry, University of Oxford, United KIngdom (UK).
The high tendency for errors during cell division, which originates chromosomal anomalies, is currently one of the biggest limitations in the use of these cells.
"To overcome this limitation we must understand why such mistakes occur. Beyond the importance of this discovery, we are now looking at other structures important in cell division in order to have a more holistic vision of all stem cell mitotic machinery. We want to be able to reverse the tendency to erroneous divisions."
Raquel Oliveira, Principal Investigator, IGC, Oeiras, Portugal.
This research brings new perspective into understanding cell division fidelity, pointing our possible causes for the presence of anomalies. This can greatly impact therapies being developed in the field of regenerative medicine.
Abstract
Pluripotent stem cells (PSCs) are central to development as they are the precursors of all cell types in the embryo. Therefore, maintaining a stable karyotype is essential, both for their physiological role as well as for their use in regenerative medicine. Karyotype abnormalities in PSCs in culture are common but the underlying causes remain unknown. To gain insight, we explore the composition of the centromere and kinetochore in human embryonic and induced PSCs. Centromere function depends on CENP-A nucleosome-defined chromatin. We show that while PSCs maintain abundant pools of CENP-A, CENP-C and CENP-T, these essential centromere components are strongly reduced at stem cell centromeres. Outer kinetochore recruitment is also impaired to a lesser extent, indicating an overall weaker kinetochore while the inner centromere protein Aurora B remains unaffected. We further show that, similar to differentiated human cells, CENP-A chromatin assembly in PSCs requires transition into G1 phase. Finally, reprogramming experiments indicate that reduction of centromeric CENP-A levels is an early event during dedifferentiation, coinciding with global chromatin remodelling. Our characterization of centromeres in human stem cells suggests a possible link between impaired centromere function and stem cell aneuploidies.
Authors
Inês Milagre, Carolina Pereira, Raquel A. Oliveira and Lars E. T. Jansen.
Acknowledgements
The authors thank José Silva (Cambridge Stem Cell Institute) for the pB-CAG-Dest-pA-pgk-bsd and pBASE plasmids, Don W. Cleveland (UCSD) for CENP-E antibodies and João Mata (IGC) for technical support. We would like to thank the members of the Jansen and Oliveira laboratories for helpful discussions and acknowledge the technical support of IGC's Advanced Imaging Facility (AIF-UIC), which is supported by the national Portuguese funding ref no. PPBI-POCI-01-0145-FEDER-022122, co-financed by Lisboa Regional Operational Programme (Lisboa 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) and Fundação para a Ciência e a Tecnologia (FCT; Portugal). We would like to thank the Flow Cytometry Facility of Instituto Gulbenkian de Ciência for their services and assistance.
Return to top of page.
| |
|
Nov 9 2020 Fetal Timeline Maternal Timeline News
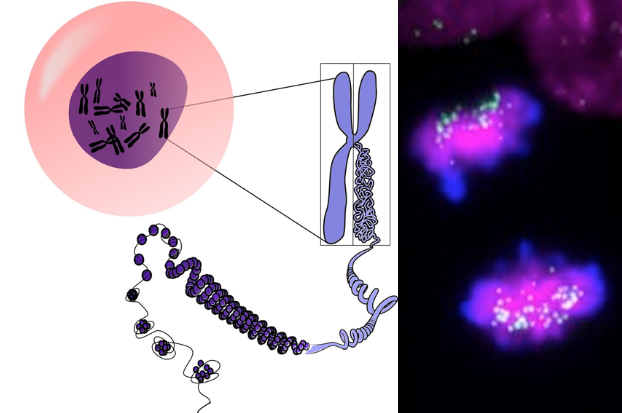 LEFT Illustration of 2 Chromosomes joined in X configuration, at the CENTROMEREunraveling into strings of DNA. RIGHT Actual human induced pluripotent stem cells. Centromeres are GREEN Chromosomes are MAGENTA. CREDIT Carolina Pereira and Inez Milagre.
|



