|
|
Developmental Biology - Pluripotent Stem Cells
Making Stem Cells from Patient Airway Cells
For the first time, researchers are creating airway stem cells by reprogramming blood cells from patients...
For the first time, researchers have successfully created airway basal stem cells in the lab from induced pluripotent stem cells. They've done this by reprogramming blood cells taken from patients. Airway basal cells are defined as stem cells from airways because they can regenerate airway epithelial cells in response to injury.
This study may help increase research on diseases impacting our airways such as COVID-19, influenza, asthma and cystic fibrosis.
Led by researchers at the Center for Regenerative Medicine at Boston Medical Center and Boston University (CReM), in collaboration with The University of Texas Health Science Center at Houston (UTHealth), these findings represent a critical first step towards airway regeneration, which will advance the field of regenerative medicine as it relates to airway and lung diseases.
Published in Cell Stem Cell, this novel study outlines how to efficiently generate and purify large quantities of airway basal stem cells using patient cells. This allows for development of individual, disease-specific airway basal stem cells in the lab that can be used to develop disease models, ultimately to lead to drug development and a platform on which targeted drug approaches can be tested. This study's findings and developed cell lines will be shared freely given the CReM's "Open Source Biology" philosophy, sharing information and findings that will help advance science across the globe.
"These results could lead to a better understanding, and therefore treatments for, a variety of airway diseases,"
Shingo Suzuki PhD, co-first author and post-doctoral researcher, UTHealth.
"Simply put, we have developed a way to reproduce patient-specific airway basal cells in labs - the ultimate goal being able to regenerate the airway of patients with airway diseases.
If we could make pluripotent stem cells using a sample from a patient who has cystic fibrosis, correct the mutation and replace the defective airway cells with corrected airway basal cells — that are otherwise genetically identical — we might eventually be able to cure the disease, and other diseases in the future using this same technology."
Finn Hawkins MB BCh, Pulmonologist, physician-scientist, Boston Medical Center, Principal Investigator of CReM and Pulmonary Center, and study first author.
• Induced pluripotent stem cells are master stem cells that can produce any cell or tissue in the human body.
• They are created by reprogramming a human sample, such as a drop of blood, into a population of cells that are similar to embryonic stem cells, which includes the ability to form different cell types within organs.
• For this study, researchers established methods to generate airway stem cell basal cells, in the laboratory. These are an important cell types found in human airways that maintain the cells lining of our airways, including cells that make mucus and cells that propel that mucus up and out of our lungs.
Researchers first engineered induced pluripotent stem cells with a gene sequence encoded with fluorescent protein allowing them to see, track and purify basal cells wherever they are present.
Researchers then read studies on the human embryo to determine how basal cells form as lungs develop. By manipulating induced pluripotent stem cells with a series of steps aimed to simulate what happens during lung development, researchers successfully generated cells highly similar to human airway basal cells in terms of: (1) appearance, (2) the genes these cells expressed — and more importantly, (3) their ability to both proliferate and form other airway cell types.
These cells, now termed ibasal cells, regenerated an airway in a live rodent.
The resulting ibasal cells, made from patients with a variety of lung diseases, were able to model all the airway diseases affecting those patients, including:
• Mucus Metaplasia characteristic of Asthmatic airways
• Chloride Channel Dysfunction causing Cystic Fibrosis
• Defects in beating cilia causing the disease Primary Ciliary Dyskinesia.
This approach will enable future opportunities for study of genetic changes, and how to reverse them in order to cure these diseases in humans.
From a practical perspective, ibasal cells grow well in special culture conditions in a lab, allowing them to be made in large numbers, and patient specific basal cells can be grown, frozen for future work, and shared with the broader research community.
"We demonstrated the potential of these ibasal cells to model both human development and disease, providing evidence of their capacity to regenerate airway epithelium.
We expect this will be a significant breakthrough and will contribute to new insights and treatment options for airway diseases, as our results overcame several important hurdles currently limiting progress in the field."
Finn J. Hawkins MBBCh, Principal Investigator, Center for Regenerative Medicine and The Pulmonary Center; primary research in human lung development and disease using human induced pluripotent stem cells (iPSCs).; currently using iPSC technology to study airway biology with a focus on Cystic Fibrosis, Boston, MA, USA.
Abstract
Highlights
• Directed differentiation of human iPSCs generates airway basal cells (?iBCs?)
• iBCs self-renew and display multipotent differentiation in vitro and in vivo
• By single-cell RNA-seq, iBCs are highly similar to adult primary airway basal cells
• iBCs enable modeling of acquired and genetic airway diseases
Summary
The derivation of tissue-specific stem cells from human induced pluripotent stem cells (iPSCs) would have broad reaching implications for regenerative medicine. Here, we report the directed differentiation of human iPSCs into airway basal cells (?iBCs?), a population resembling the stem cell of the airway epithelium. Using a dual fluorescent reporter system (NKX2-1GFP;TP63tdTomato), we track and purify these cells as they first emerge as developmentally immature NKX2-1GFP+ lung progenitors and subsequently augment a TP63 program during proximal airway epithelial patterning. In response to primary basal cell medium, NKX2-1GFP+/TP63tdTomato+ cells display the molecular and functional phenotype of airway basal cells, including the capacity to self-renew or undergo multi-lineage differentiation in vitro and in tracheal xenografts in vivo. iBCs and their differentiated progeny model perturbations that characterize acquired and genetic airway diseases, including the mucus metaplasia of asthma, chloride channel dysfunction of cystic fibrosis, and ciliary defects of primary ciliary dyskinesia.
Authors
Finn J. Hawkins, Shingo Suzuki,Mary Lou Beermann, Cristina Barill, Ruobing Wang, Carlos Villacorta-Martin, Andrew Berical, J. C. Jean, Jake Le Suer, Taylor Matte Chantelle Simone-Roach, Yang Tang, Thorsten M. Schlaeger, Ana M. Crane, Nadine Matthias, Sarah X. L. Huang, Scott H. Randell, Joshua Wu, Jason R. Spence, Gianni Carraro, Barry R. Stripp, Andras Rab, Eric J. Sorsher, Amjad Horani, Steven L. Brody, Brian R. Davis and Darrell N. Kotton.
Acknowledgements
Funding for this study was provided by the Cystic Fibrosis Foundation and National Heart, Lung, and Blood Institute.
About Boston Medical Center
Boston Medical Center (BMC) is a private, not-for-profit, 514-bed, academic medical center that is the primary teaching affiliate of Boston University School of Medicine. It is the largest and busiest provider of trauma and emergency services in New England. BMC offers specialized care for complex health problems and is a leading research institution, receiving more than $166 million in sponsored research funding in fiscal year 2019. It is the 13th largest funding recipient in the U.S. from the National Institutes of Health among independent hospitals. In 1997, BMC founded Boston Medical Center Health Plan, Inc., now one of the top ranked Medicaid MCOs in the country, as a non-profit managed care organization. Boston Medical Center and Boston University School of Medicine are partners in Boston HealthNet - 12 community health centers focused on providing exceptional health care to residents of Boston. For more information, please visit http://www.bmc.org.
.
Return to top of page.
| |
|
Nov 10 2020 Fetal Timeline Maternal Timeline News
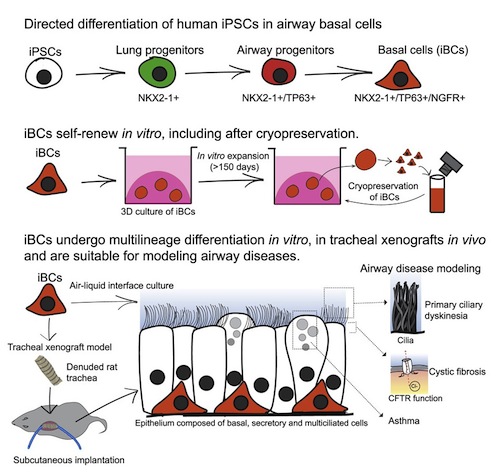 iBasal cells (iBCs) are stem cells created to regenerate and replace damaged cells lining breathing airways. CREDIT Center for Regenerative Medicine, Boston University and Boston Medical Center.
|



