|
|
Developmental Biology - Chromosome
Chromosomes Look Different Than You Think!
High-resolution, 3D images of human chromosomes in single cells reveal how DNA structure is influenced by its function...
In high school textbooks, human chromosomes are pictured as wonky hotdogs stuck together at some point just before splitting apart to create a new cell. But such images are far from accurate.
"Ninety percent of the time, chromosomes don't exist in this state."
Jun-Han Su PhD, Howard Hughes Medical Institute, Department of Chemistry and Chemical Biology; Department of Physics, Harvard University, Cambridge; Department of Molecular and Cellular Biology, Harvard University, Cambridge, Massasschusetts, USA, and first author.
Last year, before Su graduated with his PhD, he and three doctural candidates in the Graduate School of Arts and Sciences: Pu Zheng, Seon Kinrot and Bogdan Bintu, captured high-resolution 3D images of human chromosomes — which house our DNA.
Now, these images may provide evidence to change those Xs into more complex and far more accurate symbols. This will not only teach the next generation of scientists, but help current scientists unravel how chromosome structure influences chromosome function.
All living things, humans included, create new cells to replace those too damaged or worn-out to function. To do that, cells divide and replicate their DNA which is wrapped in a compicated network. Extended in a straight line, the DNA of a single cell can reach six feet in length, all of which is tightly wrapped to fit within a cell nucleus.
Just one mistake copying or re-winding that genetic material could cause genes to mutate or malfunction.
Zooming in close enough to see chromatin structure is hard. But looking at both structure and function is harder still. In their paper published August 2020 in Cell, Xiaowei Zhuang PhD and her team reported this new method they used to capture the structure and behavior of chromatin. Zhuang is best known for her work in the development of Stochastic Optical Reconstruction Microscopy (STORM), a super-resolution fluorescence microscopy method.
Zhuang with her team, has determined how structure influences behavior to either maintain proper function or possibly cause disease.
"It's quite important to determine 3D organization to understand molecular mechanisms underlying DNA organization. Also, to understand how this organization regulates genome function."
Xiaowei Zhuang PhD, the David B. Arnold, Jr. Professor of Science, Professor of Chemistry and Chemical Biology; Professor of Physics, Harvard University; and Investigator, the Howard Hughes Medical Institute; received the 2019 Breakthrough Prize in Life Sciences for developing super-resolution imaging techniques allowing visualization of small structures within living cells.
With their new high-resolution 3D imaging method, the team started building a chromosomal map from wide-lens images for all 46 chromosomes, including close-ups on one section of one chromosome. To image something this small, they captured connecting dots ("genomic loci") along each DNA chain. By connecting dots, they formed a comprehensive picture of chromatin structure.
However, Zhuang explains, the number of dots that could be imaged and identified was limited by the number of colors they could capture: three. Three dots can't make a comprehensive picture.
So, Zhuang and her team came up with a sequential approach: Image three different loci, stop the signal, and then image another three in rapid succession. With this technique, each dot gets two identifying marks: colors and location.
"Now we actually have 60 loci simultaneously imaged and localized and - importantly - identified," explains Zhuang.
Still, to cover the whole genome, they needed thousands more, so they turned to a language that's already used to organize and store huge amounts of information: binary. By imprinting binary barcodes on different chromatin loci, they could image far more loci and decode identities later. For example, a molecule imaged in round one but not round two gets a barcode starting with "10." With 20-bit barcodes, the team could differentiate 2,000 molecules in just 20 rounds of imaging. "In this combinatorial way, we can increase the number of molecules imaged - and identify each much more rapidly," says Zhuang.
With this technique, the team imaged about 2,000 chromatin loci per cell, more than a ten-fold increase over previous work and enough to form a high-resolution image of what the structure of chromosomes looks like in its native habitat. They also imaged transcription activity (when RNA replicates genetic material from DNA) and nuclear structures like nuclear speckles and nucleoli.
With their 3D Google Maps of the genome, they could start to analyze how the structure shifts over time and how those territorial movements help or hurt cell division and replication.
Researchers already know chromatin is broken into different areas and domains (as in 'desert' versus 'city'). But what terrains look like in different cell types and how they function - is still unknown. With these images, Zhuang and team determined areas with lots of genes ("gene-rich") tended to flock to similar areas on any chromosome. But, areas with few genes ("gene-poor") only come together if they share the same chromosome. One theory is that gene-rich areas, which are active sites for gene transcription, come together like a factory to enable more efficient production.
While more research is needed before confirming this theory, one thing is now certain: local chromatin environment impacts transcription activity.
Structure does influence function!
The team discovered no two chromosomes look the same, even in cells otherwise identical. To discover what each chromosome looks like in every cell in the human body will take far more work than one lab can do alone.
"It's not going to be possible to build just on our work. We need to build on many, many labs' work in order to build comprehensive understanding."
Xiaowei Zhuang PhD
Abstract Highlightst
• Massively multiplexed FISH enables mapping chromatin structure at genome scale
• Multimodal high-throughput imaging places chromatin structure in functional context
• Trans-chromosome or long-range interactions occur preferentially among active chromatin
• Transcription activity correlates with local enrichment of compartment A chromatin
Summary
The 3D organization of chromatin regulates many genome functions. Our understanding of 3D genome organization requires tools to directly visualize chromatin conformation in its native context. Here we report an imaging technology for visualizing chromatin organization across multiple scales in single cells with high genomic throughput. First we demonstrate multiplexed imaging of hundreds of genomic loci by sequential hybridization, which allows high-resolution conformation tracing of whole chromosomes. Next we report a multiplexed error-robust fluorescence in situ hybridization (MERFISH)-based method for genome-scale chromatin tracing and demonstrate simultaneous imaging of more than 1,000 genomic loci and nascent transcripts of more than 1,000 genes together with landmark nuclear structures. Using this technology, we characterize chromatin domains, compartments, and trans-chromosomal interactions and their relationship to transcription in single cells. We envision broad application of this high-throughput, multi-scale, and multi-modal imaging technology, which provides an integrated view of chromatin organization in its native structural and functional context.
Authors
Jun-Han Su, Pu Zheng, Seon S. Kinrot, Bogdan Bintu and Xiaowei Zhuang.
Acknowledgements
FUNDER
National Institutes of Health, Harvard Molecules, Cells and Organisms Training Program, Howard Hughes Medical Institute.
Return to top of page.
| |
|
Nov 19 2020 Fetal Timeline Maternal Timeline News
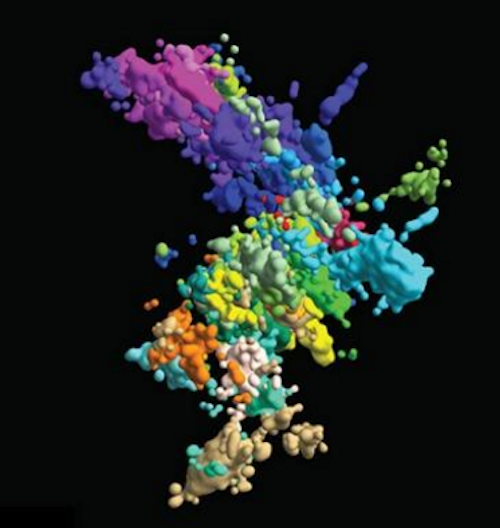 This multicoloured image of chromatin was created using multiplexed fluorescence in situ hybridization and super-resolution microscopy. CREDIT Xiaowei Zhuang lab.
|



