|
|
Developmental Biology - Stem Cells
Solving The Mystery of Embryonic Stem Cell Change
Study of neural crest cells could boost stem cell therapy research into craniofacial malformations such as cleft palate and aggressive cancers like melanoma and neuroblastoma...
Last year, researchers at the University of California, Riverside, identified the early origins of neural crest cells in chick embryos. These embryonic cells in vertebrates (humans included) travel throughout the body generating many cell types. Now, using a human model, researchers have figured out when neural crest cells will acquire distinct molecular and functional change.
The study, published in Stem Cell Research, gives new insight into how neural crest cells form, outlining transient stages in cell development. It also reveals how the neural crest cell lineage is distinct from pluripotent stem cells.
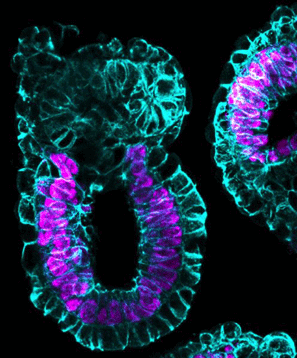
The neural crest is an important embryonic cell population. In a developing embryo, it generates neurons, glia, melanocytes, along with cells that make up bone and cartilage.
Errors in development are linked to a wide range of pathologies, from craniofacial malformations such as cleft palate — to aggressive cancers like melanoma and neuroblastoma.
This study used a robust human model of neural crest formation to demonstrate a fast transition from the pluripotent stem cell state to the neural crest precursor state. According to this model, a sequential loss of pluripotency markers occurs during the pluripotent stem cell state as cells transition to neural crest cells.
"Defining the molecular signature required for neural crest formation, helps us understand human neural crest pathologies and develop diagnostic/therapeutic efforts. Knowledge of the precise time and molecular signals involved - for example: exactly when neural crest cells acquire the potential to form jaw and teeth - will enable replication and modifying this potential in stem cells for therapies aiding regenerative craniofacial repairs.
We address the precise timing when pluripotent stem cells diverge toward neural crest cell lineage by exploring distinct molecular and functional attributes of early neural crest cells - something never before established. We also identified unique molecular signatures during transition of neural crest cells from pluripotent stem cells."
Maneeshi S. Prasad PhD, assistant project scientist, Martin I. Garcia-Castro laboratory; associate professor, Biomedical Sciences, Univercity of California, Riverside - School of Medicine. California, USA.
Researchers created a high-resolution map of gene expression and epigenetic changes showing well-defined stages of neural crest formation. They believe it will be a valuable resource for scientists identifying and studying various genes involved in human neural crest formation.
Neural crest cells have been thought to originate in the ectoderm, which is one of three germ cell layers forming in the earliest stages of embryo development.
But, their abiliity to initiate bone and tooth forming cells is in conflict with fundamental concepts in developmental/stem cell biology.
Garcia-Castro points out their study also established a novel in-vitro test to determine differentiation capacity of specific neural crest cells into germ cell layers such as mesoderm and endoderm.
"Our work demonstrates how neural crest cells depart from a pluripotent stem cell state soon after Wnt signaling activates — an ancient, evolutionary pathway regulating crucial aspects of cell behavior.
Importantly, using our novel specification test we found prospective neural crest cells lose the potential for mesodermal and endodermal characteristics of pluripotent stem cells — just hours upon induction."
Martin I. Garcia-Castro PhD,
School of Medicine Division of Biomedical Sciences, University of California, Riverside, USA PhD; Associate Professor, Biomedical Sciences, Univercity of California, Riverside - School of Medicine. California, USA.
Abstract Highlights
• Early molecular and stochiometric changes in NC and ES genes define a prospective NC cell state.
• Human NC specification requires ?-catenin dependent activation of early NC genes.
• Prospective NC cells have restricted stem cell potential distinct from pluripotent ES cells.
Abstract
Neural crest cells are an embryonic multipotent stem cell population. Recent studies in model organisms have suggested neural crest cells are specified earlier than previously thought, at blastula stages. However, the molecular dynamics of early neural crest specification, and functional changes from pluripotent precursors to early specified NC, remain to be elucidated. In this report, we utilized a robust human model of cranial neural crest formation to address the distinct molecular character of the earliest stages of neural crest specification and assess the functional differences from its embryonic stem cell precursor. Our human neural crest model reveals a rapid change in the epigenetic state of neural crest and pluripotency genes, accompanied by changes in gene expression upon Wnt-based induction from embryonic stem cells. These changes in gene expression are directly regulated by the transcriptional activity of ?-catenin. Furthermore, prospective cranial neural crest cells are characterized by restricted stem cell potential compared to embryonic stem cells. Our results suggest that human neural crest induced by Wnt/?-catenin signaling from human embryonic stem cells rapidly acquire a prospective neural crest cell state defined by a unique molecular signature and endowed with limited potential compared to pluripotent stem cells.
Authors
Maneeshi S. Prasad, Rebekah M.Charney, Lipsa J.Patel Martin and I.Garcia-Castro.
Acknowledgements
The research was funded by the National Institutes of Health.
The title of the research paper is "Distinct molecular profile and restricted stem cell potential defines the prospective human cranial neural crest from embryonic stem cell state.
The University of California, Riverside (http://www.ucr.edu) is a doctoral research university, a living laboratory for groundbreaking exploration of issues critical to Inland Southern California, the state and communities around the world. Reflecting California's diverse culture, UCR's enrollment is more than 24,000 students. The campus opened a medical school in 2013 and has reached the heart of the Coachella Valley by way of the UCR Palm Desert Center. The campus has an annual statewide economic impact of almost $2 billion. To learn more, email news@ucr.edu.
Return to top of page.
| |
|
Nov 20 2020 Fetal Timeline Maternal Timeline News
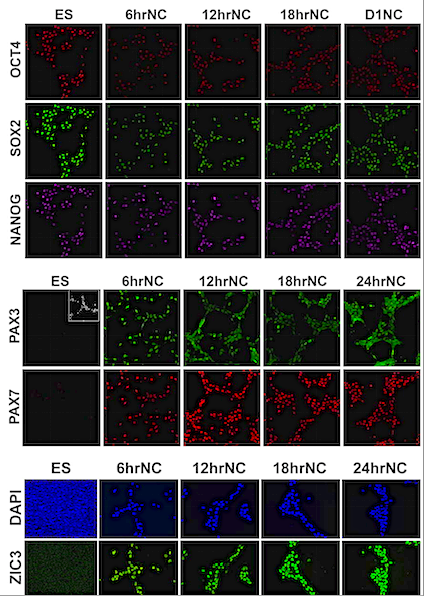 Pictured is a diagram of cell differentiation over time in neural crest cells of chick embryos. Research at University of California, Riverside has identified when neural crest cells undergo unique molecular changes. The period investigated is of particular importance to the formation of teeth and bones. CREDIT Martin I. Garcia-Castro laboratory, UC Riverside.
|