|
|
Developmental Biology - Skull & Facial Growth
Craniofacial Development
New insights into the origins of facial birth defects...
Mount Sinai researchers reveal how FGFs regulate craniofacial development in utero, which can sometimes lead to birth defects such as cleft lip or palate.
A new study published November 12 in Cold Spring Harbor Laboratory's research journal Genes and Development, reports that fibroblast growth factors (FGFs), a family of macromolecules (very large molecules, such as proteins), are affecting biological processes beyond simple cell signaling. They believe FGFs particularly target cells adhesion to one another, and might play a pivotal role in regulating a host of other pathologies.
FGFs bind to and activate four different molecules called receptor tyrosine kinases (RTKs) on the surface of the cell.
These RTKs, in turn, trigger an established signaling pathway that influences cell behaviors such as proliferation, death, and migration. Improper activation of these receptors and aberrant signaling within these so-called signal transduction pathways, is now also linked, to premature bone formation in newly forming brain sutures - the fibrous joints between bones of a baby's skull - which then fuse together before the brain is fully formed, giving the infant's head a misshapen appearance.
Deregulation of FGF activity has also been linked to multiple forms of cancer.
"Through our laboratory work with mice we've found - for the first time - the unique role of signaling pathways engaged by FGF receptors in embryonic development.
These signaling mechanisms and the phenotypic consequences of their disruption, give us a better understanding of how FGFs affect mid-face closure and development of the jaw.
In the mouse, FGF receptors also affect implantation of the embryo into the uterus."
Philippe Soriano PhD, Professor of Cell, Development and Regenerative Biology, Icahn School of Medicine at Mount Sinai,New York, New York, USA, and senior author of the study>
Over the years, Dr. Soriano's laboratory has played a key role in unraveling the mechanism of RTK by using genetic approaches in mice as a model system.
Mount Sinai researchers also broke new ground by uncovering how RTKs may function beyond their well-known roles in cell signaling.
By engineering mutant mice that express receptors unable to engage the classic signaling pathways, Mount Sinai researchers were also able to identify how FGFs regulate cell adhesion, a process by which cells stick to each other or to the extracellular matrix, which provides structural and biochemical support for surrounding cells.
"We always thought all FGF activities depend on the typical established signaling pathways. However, we were able to identify new signaling outputs that seem to function independent of FGF signal transduction pathways. One of those outputs is - cell adhesion."
Philippe Soriano PhD.
Dr. Soriano and his team are continuing to investigate not only how FGF receptors work on the surface of the cell - which is established science — but in the cell, to modify how cells stick to each other or to the extracellular matrix. Giving impetus to their work is the fact that knowing precisely how FGFs regulate cell adhesion could open a valuable window for scientists to a process believed to underlie the development of many types of cancer.
"Perhaps more importantly, we've created surprising new investigative channels with regard to cell adhesion and signaling pathways. We now want to know if there are additional biological processes at play that could bring us closer to inhibiting these pathways and prevent diseases in which FGFs and their receptors are believed complicit."
Philippe Soriano PhD.
Abstract
FGFs are key developmental regulators that engage a signal transduction cascade through receptor tyrosine kinases, prominently engaging ERK1/2 but also other pathways. However, it remains unknown whether all FGF activities depend on this canonical signal transduction cascade. To address this question, we generated allelic series of knock-in Fgfr1 and Fgfr2 mouse strains, carrying point mutations that disrupt binding of signaling effectors, and a kinase dead allele of Fgfr2 that broadly phenocopies the null mutant. When interrogated in cranial neural crest cells, we identified discrete functions for signaling pathways in specific craniofacial contexts, but point mutations, even when combined, failed to recapitulate the single or double null mutant phenotypes. Furthermore, the signaling mutations abrogated established FGF-induced signal transduction pathways, yet FGF functions such as cell-matrix and cell-cell adhesion remained unaffected, though these activities did require FGFR kinase activity. Our studies establish combinatorial roles of Fgfr1 and Fgfr2 in development and uncouple novel FGFR kinase-dependent cell adhesion properties from canonical intracellular signaling.
Authors
Ayan T. Ray, Pierre Mazot, J. Richard Brewer, Catarina Catela, Colin J. Dinsmore and Philippe Soriano.
Acknowledgements
About the Mount Sinai Health System
The Mount Sinai Health System is New York City's largest academic medical system, encompassing eight hospitals, a leading medical school, and a vast network of ambulatory practices throughout the greater New York region. Mount Sinai is a national and international source of unrivaled education, translational research and discovery, and collaborative clinical leadership ensuring that we deliver the highest quality care - from prevention to treatment of the most serious and complex human diseases. The Health System includes more than 7,200 physicians and features a robust and continually expanding network of multispecialty services, including more than 400 ambulatory practice locations throughout the five boroughs of New York City, Westchester, and Long Island. The Mount Sinai Hospital is ranked No. 14 on U.S. News & World Report's "Honor Roll" of the Top 20 Best Hospitals in the country and the Icahn School of Medicine as one of the Top 20 Best Medical Schools in the country. Mount Sinai Health System hospitals are consistently ranked regionally by specialty by U.S. News & World Report.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.
Return to top of page.
| |
|
Nov 23 2020 Fetal Timeline Maternal Timeline News
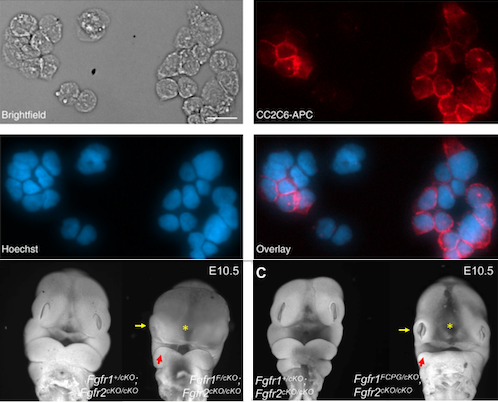 TOP 2 Rows: Stained Cells Isolation of single cells for - Omics a Space Microscopy images [left-to-right; anti-CD47 antibody (RED); Hoechst for nuclear staining (BLUE); Overlay (blue and red) of Immunofluorescent stained cells on a DISCO device. b Fluorescence microscopy images of cells stained identically to (A) BEFORE (left) and AFTER (right) lysis of a single KO cell. CREDIT Tetyana Milojevic. BOTTOM ROW: Frontal facial view of DAPI-stained E10.5 Fgfr1F/cKO; Fgfr2 cKO/cKO embryos compared to control Fgfr1+/cKO; Fgfr2+/cKO embryos. See article in Genes & Development..
|



