|
|
Developmental Biology - Preeclampsia
Preeclampsia and Vitamin D
According to the World Health Organization, preeclampsia affects between 2% to 8% of pregnancies...
A single gene may correlate to vitamin D status during pregnancy and bring on preeclampsia, which can cause serious, sometimes fatal, complications in the mother — and baby. Among risk factors, such as obesity and diabetes, vitamin D deficiency during pregnancy has been associated with its increased risk.
Now, a team of researchers at the Medical University of South Carolina (MUSC) has found that expression of a set of genes, previously studied in early onset and severe preeclampsia, is significantly affected by vitamin D status during late-stage pregnancy. Their research appears in an article in Pregnancy Hypertension.
Exactly how preeclampsia develops has been unclear. However, recent evidence points to poor development of placental blood vessels that otherwise would be nourishing the fetus. Their deficiency leads to hypertension and several other complications for the mother. Today, the only cure for preeclampsia is to deliver the fetus, dangerous for the infant if too early in its development.
In a previous study supported by the South Carolina Clinical & Translational Research Institute (SCTR), and led by Kyu-Ho Lee, found the expression of three genes: NKX2-5, SAM68, and sFLT1, are highly correlated to early-onset and severe preeclampsia (EOSPE). One gene, sFLT1, is an identified marker for risk of preeclampsia. In the current study, the authors examined expression of these 3 genes in healthy pregnant women.
"Having observed the correlated expression of these genes in preeclampsia, we wanted to see their pattern of expression in normal pregnancy."
Kyu-Ho Lee MD PhD, assistant professor, Departments of Pediatrics and Obstetrics and Gynecology, MUSC.
An important regulator of blood vessel development is the protein sFLT1 - which interferes with the activity of vascular endothelial growth factor (VEGF), thus can reduce placental blood vessel growth.The amount of sFLT1 in the placenta is regulated partly by the genes SAM68 and NKX2-5, known as the "tinman" genes. So, this hypothesized NKX2-5/SAM68/sFLT1 gene "axis" may actually contribute to development of preeclampsia.
"NKX2-5 might be controlling the regulation of sFLT1 and SAM68 in such a way that in preeclampsia, the expression levels of those genes go awry and tilt the vascular development in a direction that triggers preeclampsia."
Kyu-Ho Lee MD PhD.
Vitamin D affects many aspects of a mother's health during pregnancy. To investigate how this axis of 3 genes reflected by a mother's vitamin D status, Lee's team studied placental samples from 43 pregnant women enrolled in a clinical trial organized by MUSC Health neonatologist Carol L. Wagner MD, professor in the department of Pediatrics. Half of the women received a high dose of vitamin D3 (4,000 IU/day), while the other half received a placebo. The study group included African American, Hispanic American and Caucasian American women.
In contrast to their previous study, the team did not detect significant levels of NKX2-5 in placental tissue samples before any of the healthy deliveries. A finding that marks NKX2-5 expression as an important indicator of at-risk pregnancies. However, the team did observe a strong positive correlation between SAM68 and sFLT1 in all study participants.
"The tight correlation between SAM68 and sFLT1 makes us think there is a functional relationship between these genes."
Kyu-Ho Lee MD PhD.
Interestingly, when assessed at the last visit before delivery, women who were vitamin D deficient had significantly higher expression of sFLT1 and lower expression of VEGF in their placenta than women who were vitamin D sufficient. The study thus provides novel insight into the activity of the NKX2-5/SAM68/sFLT1 gene axis in healthy pregnancies, in a diverse group of women, and how it may be affected by the vitamin D status of the mother. Lee believes this understanding will improve the standard of care for treating preeclampsia.
"These results raise the possibility that vitamin D somehow directly regulates sFLT1 and/or SAM68 expression at some level. However, at this point, we haven't addressed their exact molecular relationship."
The team's current findings contribute to a basic biological understanding of the development of pregnancy with regards to preeclampsia, and believes this understanding will improve the standard of care for treating preeclampsia.
Abstract Objectives
The goal of this study was to determine if an axis of placental gene expression associated with early onset and severe preeclampsia (EOSPE) was operative in term pregnancy and correlated with vitamin D sufficiency.
Methods
qPCR analysis of NKX2-5, SAM68, sFLT1 and membrane bound VEGFR1/FLT1 mRNA expression was conducted in placentas from 43 subjects enrolled in a vitamin D3 pregnancy supplementation trial. Pair-wise rank order correlations between patient-specific gene expression levels were calculated, and their relationship to maternal 25(OH)D status was assessed by a two-sample Wilcoxon test. Additionally, we probed the mechanistic link between SAM68 and sFLT1 using siRNA depletion in a human trophoblast cell line model.
Results
Positive and highly significant correlations were found between SAM68 vs. sFLT1 and SAM68 vs. FLT1 expression levels, as were significant and differential correlations between the expression of these genes and perinatal 25(OH)D status. The variability when stratified by race/ethnicity was qualitatively distinct from those previously observed in EOSPE. Mechanistic studies confirmed a functional role for SAM68 protein in the regulation of sFLT1 expression. NKX2-5 expression was not significantly correlated with sFLT1 or SAM68 expression in these samples, suggesting that its expression may be significant at earlier stages of pregnancy or be restricted to pathological settings.
Conclusions
These data further support our overarching hypothesis that SAM68 expression is a key determinant of VEGFR1 isoform expression in the placenta, and provide additional insights into how this gene pathway may be differentially deployed or modified in normal and pathological pregnancies.
Authors
Oyindamola Awe, James M. Sinkway, Rebecca P. Chow, Quentell Wagener, Elizabeth V. Schulz, Jeremy Y. Yu, Paul J. Nietert, Carol L. Wagner and Kyu-Ho Lee.
Return to top of page.
| |
|
Nov 24 2020 Fetal Timeline Maternal Timeline News
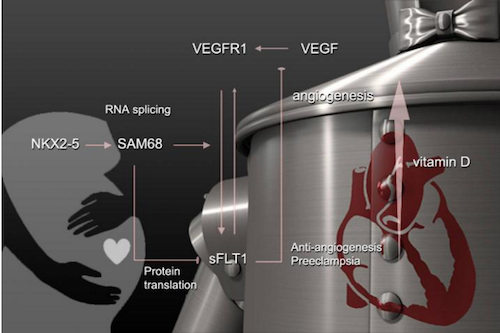 Illustration shows the relationship between an axis of genes that includes NKX2-5 (the tinman gene) and preeclampsia. Angiogenesis is the development of new blood vessels. VEGFR1 = vascular endothelial growth factor receptor 1. CREDIT Medical University of South Carolina.
|



