|
|
Developmental Biology - Brain Size: Primary Microcephaly
How RRP7A Gene Affects Brain Size
Mutations in both copies of the RRP7A gene are critical to proliferation and formation of new neurons...
Research under Lars Allan Larsen and Soren Tvorup Christensen at the University of Copenhagen (UCPH), Denmark, has identified an important step in the development of our cerebral cortex — that part of our brain playing a key role in our attention, perception, awareness, thought, memory, language, and consciousness.
They have identified that mutations in the Rrp7a gene lead to defects in stem cell generation. RRP7A is typically localized in centrosomes which connect DNA pairs just before cell division (mitosis).
Their research results are published in the journal Nature Communications.
In a large family in which 10 of the children were born with congenital primary microcephaly, (a rare brain disorder where the cerebral cortex is reduced in size), the researchers found mutations in both copies of the RRP7A gene. Using stem cell cultures and zebrafish (for modelling the disorder) RRP7A was shown to be critical in brain stem cell proliferation and the formation of new neurons. The research may explain why this mutation affects only the brain and no other tissues or organs.
"Our discovery is surprising as it reveals an unknown mechanisms in brain development. Additionally, it highlights the value in research on rare disorders, important for both patients and families. The discovery is also beneficial just as new knowledge about human biology."
Lars Allan Larsen, Professor, Department of Cellular and Molecular Medicine, University of Copenhagen, Copenhagen, Denmark.
Researchers found the RRP7A mutation affects the function of the primary cilia, antenna-like structures on the surface of cells that register environmental cues and control the formation of new neurons in the developing brain.
"These results open a new avenue in understanding how primary cilia control developmental processes; and, how certain mutations within these antenna-like structures compromise formation of tissues and organs during development.
We have initiated a series of investigations into mechanisms by which RRP7A regulates ciliary signaling and how defects in signaling lead to brain malformation and cognitive disorders.
Søren Tvorup Christensen, Professor,
Department of Biology, University of Copenhagen, Copenhagen, Denmark
.
Abstract
Primary microcephaly (MCPH) is characterized by reduced brain size and intellectual disability. The exact pathophysiological mechanism underlying MCPH remains to be elucidated, but dysfunction of neuronal progenitors in the developing neocortex plays a major role. We identified a homozygous missense mutation (p.W155C) in Ribosomal RNA Processing 7 Homolog A, RRP7A, segregating with MCPH in a consanguineous family with 10 affected individuals. RRP7A is highly expressed in neural stem cells in developing human forebrain, and targeted mutation of Rrp7a leads to defects in neurogenesis and proliferation in a mouse stem cell model. RRP7A localizes to centrosomes, cilia and nucleoli, and patient-derived fibroblasts display defects in ribosomal RNA processing, primary cilia resorption, and cell cycle progression. Analysis of zebrafish embryos supported that the patient mutation in RRP7A causes reduced brain size, impaired neurogenesis and cell proliferation, and defective ribosomal RNA processing. These findings provide novel insight into human brain development and MCPH.
Authors
Muhammad Farooq, Louise Lindbaek, Nicolai Krogh, Canan Doganli, Cecilie Keller, Maren Minnich, Andrew Brus Gonzalves, Srinivasan Sakthivel, Yuan Mang, Ambrin Fatima, Vivi Sogaard Andersen, Muhammad S. Hussain, Hans Eiberg, Lars Hansen, Klaus Wilbrandt Kjaer, Jay Gopalakrishnan, Lotte Bang Pedersen, Kjeld Mollgard, Henrik Nielsen, Shahid. M. Baig, Niels Tommerup, Soren Tvorup Christensen and Lars Allan Larsen.
Acknowledgements
The authors thank Maria S. Holm, Lillian E. Rasmussen, Pernille S. Froh, Troels Askhoj Andersen, and Martin Juel Vilhelm for excellent technical assistance. This study was supported by grants from the Lundbeck Foundation (R221-2016-922), the University of Copenhagen Excellence Program for Interdisciplinary Research, the Novo Nordisk Foundation (NNF18OC0053024, NNF14OC0011535, and NNF15OC0016886), Independent Research Fund Denmark (DFF-6108-00457 and 8020-00162B), the Danish Cancer Society (R146-A9590) and the Carlsberg Foundation (CF15-0425, CF18-0532, and CF-0294) to S.T.C., L.A.L., and L.B.P.; grants from the Lundbeck Foundation (R198-2015-174) and the Danish Cancer Society (R167-A10943-17-S2) to H.N. and N.K.; a grant from the Independent Research Fund Denmark (DNRF107) to L.H.; a grant from The Lundbeck Foundation (R209-2015-2604) to C.D; a grant from the Lundbeck Foundation (2013-14290) to N.T.
This work is the result of interdisciplinary collaboration between 23 scientists from Denmark, Germany and Pakistan, and funded by The Lundbeck Foundation, Independent Research Fund Denmark, The Novo Nordisk Foundation, Carlsberg Foundation, The Danish Cancer Society, and The Excellence Program for Interdisciplinary Research at University of Copenhagen.
Return to top of page.
| |
|
Nov 26 2020 Fetal Timeline Maternal Timeline News
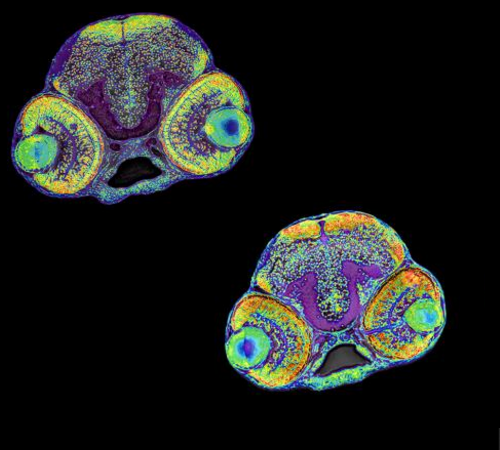 The research team used stem cells and zebrafish to investigate the role of RRP7A in brain development. This picture shows the brain of a normal zebrafish larvae (top left) and a larvae with microcephaly (lower right). CREDIT Assist. Prof. Canan Doganli, University of Copenhagen.
|



