|
|
A master switch for brain development?
Scientists at the Institute of Molecular Biology in Mainz have unraveled a regulatory mechanism which explains how a single gene can drive the formation of brain cells.
Scientists at the Institute of Molecular Biology (IMB) in Mainz, Germany, have unraveled a complex regulatory mechanism which explains how a single gene can drive the formation of brain cells.
The research published in The EMBO Journal, is an important step towards understanding how the brain develops. It also suggests potential for regenerative medicine.
Neurodegenerative disorders, such as Parkinson's disease, are often characterized by an irreversible loss of brain cells (neurons). Unlike many other cell types in the body, neurons typically do not regenerate on their own, so if neurons are damaged — they stay damaged.
In order to develop new treatments for neuronal damage, research must first understand how the brain develops. Then they try and imitate that process. However, the brain being one of the most complex organs in the body, very little is understood about molecular pathways that guide its development.
Scientists like Dr. Vijay Tiwari and his group at the Institute of Molecular Biology at Johannes Gutenberg University Mainz, have therefore been investigating a single central gene in brain development, NeuroD1. This gene, found active as the brain is developing, also marks the beginning of neurogenesis - the formation of neurons.
In their research, Tiwari and his colleagues have discovered that during brain development, NeuroD1 is not only expressed in brain stem cells but appears to act as a master regulator of a large number of brain stem cell genes — causing them to become neurons.
Using a combination of approaches found in neurobiology and computational biology, researchers found NeuroD1 activity changes the epigenetic state of brain stem cell genes, turning them permanently "on".
These modification are on the histone proteins that DNA is wound around in order to compact the long strands of DNA to fit within the nucleus of a cell. The changes to the histone proteins are heritable — that is they transfer to the next generation — but do not changes the DNA sequence. Therefore, they are referred to as ‘epigenetic’ or "outside of the DNA" gene.
The genes will remain switched on even after NeuroD1 switches off as NeuroD1 creates a permanent loosening of the DNA around the histone, keeping it easily readable and therefore "active."
"Our research has shown how a single factor, NeuroD1, has the capacity to change the epigenetic landscape of a cell, resulting in gene expression that directs the generation of neurons."
Vijay Tiwari PhD, Group Leader, Institute of Molecular Biology (IMB), Mainz, Germany
"This is a significant step towards understanding the relationship between DNA sequence, epigenetic changes and cell fate. It not only sheds new light on the formation of the brain during embryonic development but also opens up novel avenues for regenerative therapy."
Abhijeet Pataskar PhD, and Johannes Jung PhD, joint first authors.
Abstract
Cell fate decisions require the deployment of distinct transcriptional programmes—how this is controlled and orchestrated is a key question from basic developmental biology to regenerative medicine. In this issue of The EMBO Journal, Pataskar and Jung et al (Pataskar et al, 2015) demonstrate how the transcription factor NeuroD1 acts genome-wide to elicit a specific neurogenic programme, including differentiation and migration. Much of that activity is due to NeuroD1 acting as a pioneer factor. NeuroD1 is able to bind its targets within repressive chromatin and can induce a more open chromatin state amenable to cell type-specific regulation.
Reference: Our brain, more than chimp's, influenced by epigenetics
http://www.uni-mainz.de/bilder_presse/IMB_neuroD1_neurogenesis.jpg
Figure 2: Diagram showing how NeuroD1 influences the development of neurons. During brain development, expression of NeuroD1 marks the onset of neurogenesis. NeuroD1 accomplishes this via epigenetic reprogramming: neuronal genes are switched on, and the cells develop into neurons. TF: transcription factor; V: ventricle; P: pial surface. Image credit: A. Pataskar/J. Jung & V. Tiwari
News & Views by Glahs A, Zinzen RP (2015). Putting chromatin in its place: the pioneer factor NeuroD1 modulates chromatin state to drive cell fate decisions. EMBO J, Nov 13, DOI: 10.15252/embj.201593324
More information about Dr. Vijay Tiwari's research can be found at http://www.imb.de/tiwari.
About the Institute of Molecular Biology gGmbH
The Institute of Molecular Biology gGmbH (IMB) is a centre of excellence in the life sciences that was established in 2011 on the campus of Johannes Gutenberg University Mainz (JGU). Research at IMB concentrates on three cutting-edge areas: epigenetics, developmental biology, and genome stability. The institute is a prime example of a successful collaboration between public authorities and a private foundation. The Boehringer Ingelheim Foundation has dedicated EUR 100 million for a period of ten years to cover the operating costs for research at IMB, while the state of Rhineland-Palatinate provided approximately EUR 50 million for the construction of a state-of-the-art building.
About the Boehringer Ingelheim Foundation
The Boehringer Ingelheim Foundation is an independent, non-profit organisation committed to the promotion of the medical, biological, chemical and pharmaceutical sciences. It was established in 1977 by Hubertus Liebrecht (1931-1991), a member of the shareholder family of the company Boehringer Ingelheim. With the PLUS 3 Perspectives Program and the Exploration Grants, the foundation supports independent group leaders. It also endows the internationally renowned Heinrich Wieland Prize as well as awards for up-and-coming scientists. In addition, the foundation pledged to donate EUR 100 million to finance the scientific running of the IMB at Johannes Gutenberg University Mainz for ten years. In 2013, the Boehringer Ingelheim Foundation donated a further EUR 50 million to Johannes Gutenberg University Mainz.
Return to top of page
|
|
|
Nov 23, 2015 Fetal Timeline Maternal Timeline News News Archive
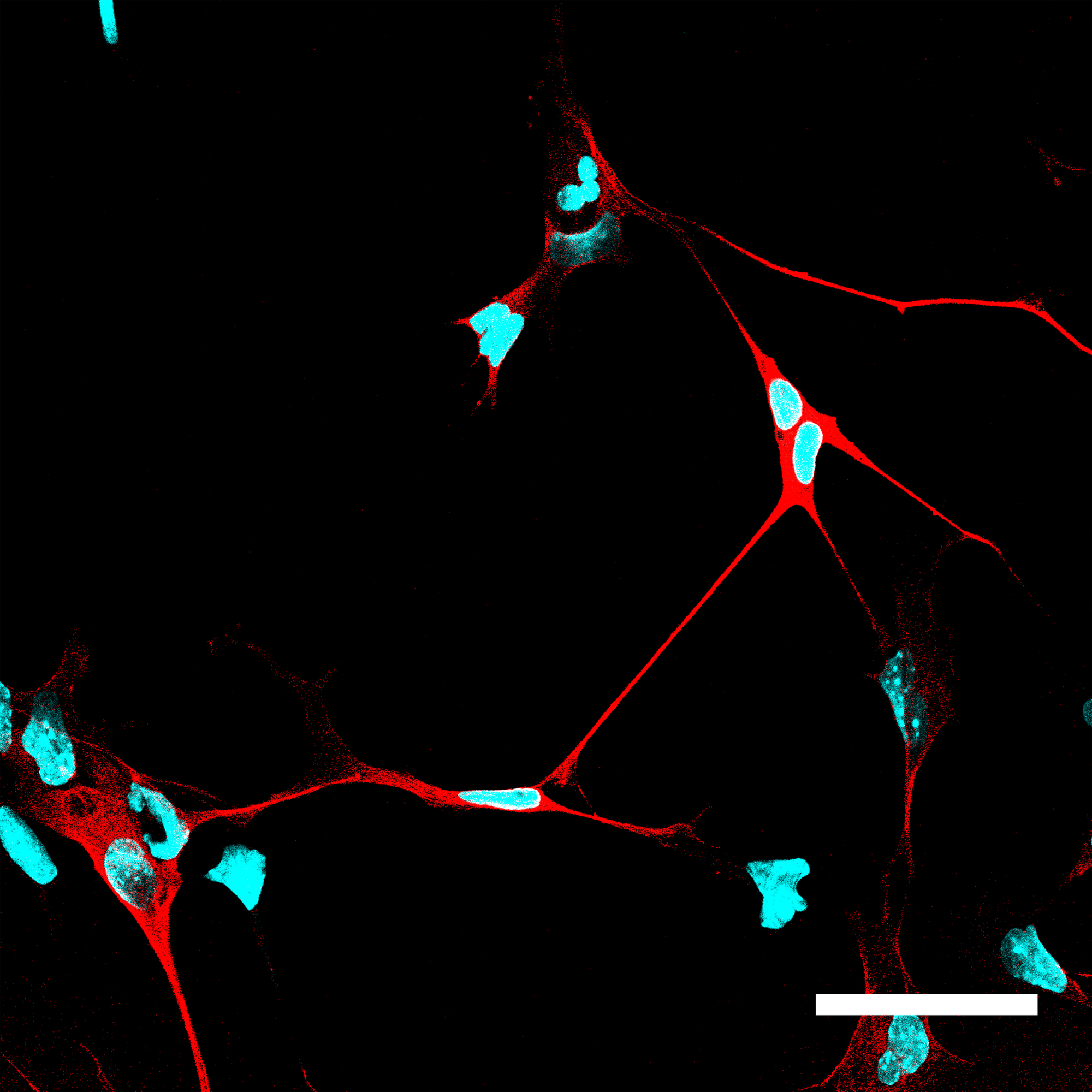
Cells in which NeuroD1 is turned on are reprogrammed to become neurons.
Cell nuclei are shown in blue and neurons, with their
characteristic long extensions, are shown in red.
Image Credit:
A. Pataskar/J. Jung & V. Tiwari
|
|
| |
|



