|
|
Welcome to The Visible Embryo, a comprehensive educational resource on human development from conception to birth.
The Visible Embryo provides visual references for changes in fetal development throughout pregnancy and can be navigated via fetal development or maternal changes.
The National Institutes of Child Health and Human Development awarded Phase I and Phase II Small Business Innovative Research Grants to develop The Visible Embryo. Initally designed to evaluate the internet as a teaching tool for first year medical students, The Visible Embryo is linked to over 600 educational institutions and is viewed by more than one million visitors each month.
Today, The Visible Embryo is linked to over 600 educational institutions and is viewed by more than 1 million visitors each month. The field of early embryology has grown to include the identification of the stem cell as not only critical to organogenesis in the embryo, but equally critical to organ function and repair in the adult human. The identification and understanding of genetic malfunction, inflammatory responses, and the progression in chronic disease, begins with a grounding in primary cellular and systemic functions manifested in the study of the early embryo.

The World Health Organization (WHO) has created a new Web site to help researchers, doctors and patients obtain reliable information on high-quality clinical trials. Now you can go to one website and search all registers to identify clinical trial research underway around the world!

|
|
| Disclaimer: The Visible Embryo web site is provided for your general information only. The information contained on this site should not be treated as a substitute for medical, legal or other professional advice. Neither is The Visible Embryo responsible or liable for the contents of any websites of third parties which are listed on this site. |
|
|

Content protected under a Creative Commons License. Commons License. |
|
| No dirivative works may be made or used for commercial purposes. |
|
|
| |
|
|
 
CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|
|
|
How cells begin to shape a living being
You were once a hollow ball of cells. Sculpting that hollow ball into an organism with layer upon layer of organs, sinew, tendons, muscle and brain, first starts when a simple ball of cells folds inwards. But why do cells start to fold into themselves? Is all cell movement based on DNA programming, or does the shape of surrounding cells also have an affect?
Using fruit fly embryos, researchers at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, identified a particular group of cells crucial to the first furrow in a simple ball of cells.
They found that the arrangement of the cells also helps determine the direction in which they will inwardly contract. Published this week in Developmental Cell, their findings are the result of a new technique in which light - a laser to be exact - is used to remotely manipulate internal cell structures.
When a fruit fly embryo is around 4 hours old, cells on its underside start contracting and pull surrounding cells into an inward fold (invagination) to form what is known as the ventral furrow. A similar inward folding occurs in the human embryo around day 13 after ovulation and begins the formation of the primitive streak, the precursor of our nervous system.
Inside each fruitfly cell is a network of fibres made of actin, a multi-functional protein found in all cells whether fruitflies and humans. Actin is important in contraction as actin fibres pull a cell's outer membrane into the cell center, changing the cell from a once round shape into a rectangle. The rectangle gets thinner and thinner as neighboring cells press upon it as they too are drawn into the embryo's center.
EMBL scientists developed a new technique using a laser to further their understanding of this process. After shining the laser on the embryo, they effectively removed the actin anchors keeping the fibres attached to the cell wall. Following this optogenetic laser treatment, individual cells could no longer contract. By applying the same technique to different parts of a developing fruitfly embryo, scientists were able to pinpoint a cluster of cells that must contract in order for the first furrow to form - and the embryo to have a normal development.
By arbitrarily changing the shape of the 'contraction' area through use of the laser light, the scientists discovered cells will only contract into a ventral furrow that is arranged in a rectangular pattern. This seems to imply that furrow shape is not soley directed by DNA programming, but is also the result of surrounding cell dynamics.
The laser approach overcomes one of the main challenges faced in studying development: it enables scientists to view manipulated effects immediately. By contrast, standard methods of turning genes on or off, requires hours or even days of waiting before such a manipulation can be observed.
"The methods we had available made it a bit like arriving at the scene of a car crash after the fact: you know something went wrong at some point, but you were not there when it happened, so you cannot tell what exactly caused the accident.
"Our new optogenetic method is more like a remote control: you turn on the laser, and see the effect within seconds."
Stefano De Renzis PhD, EMBL Heidelberg, Heidelberg, Germany, team leader.
Next, De Renzis plans to use this laser method to look at how cells in developing fruitfly embryos communicate with each other.
Abstract Highlights
•Optogenetics provides a powerful approach to control tissue morphogenesis
•Two-photon illumination allows precise patterns of optogenetic activation
•Local modulation of cell contractility reveals mechanisms of tissue invagination
•Tissue geometry constrains the cell contractile behavior driving invagination
Summary
Morphogenesis of multicellular organisms is driven by localized cell shape changes. How, and to what extent, changes in behavior in single cells or groups of cells influence neighboring cells and large-scale tissue remodeling remains an open question. Indeed, our understanding of multicellular dynamics is limited by the lack of methods allowing the modulation of cell behavior with high spatiotemporal precision. Here, we developed an optogenetic approach to achieve local modulation of cell contractility and used it to control morphogenetic movements during Drosophila embryogenesis. We show that local inhibition of apical constriction is sufficient to cause a global arrest of mesoderm invagination. By varying the spatial pattern of inhibition during invagination, we further demonstrate that coordinated contractile behavior responds to local tissue geometrical constraints. Together, these results show the efficacy of this optogenetic approach to dissect the interplay between cell-cell interaction, force transmission, and tissue geometry during complex morphogenetic processes.
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
For a first-person account of how the technique was developed, Lighting up development see http://news.embl.de/science/1511-lighting-development/
Return to top of page
|
|
|
Nov 26, 2015 Fetal Timeline Maternal Timeline News News Archive
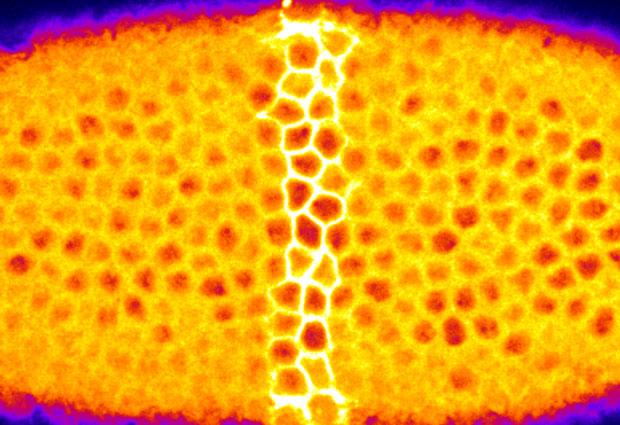
When illuminated with a laser, individual cells (bright yellow) within the fruit fly embryo
stop contracting. This new optogenetic approach gives insight into how tissues bend to form
organ systems. View movie of this process.
Image Credit:
EMBL/Giorgia Guglielmi
|
|
| |
|



