|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Jan 17, 2014
|
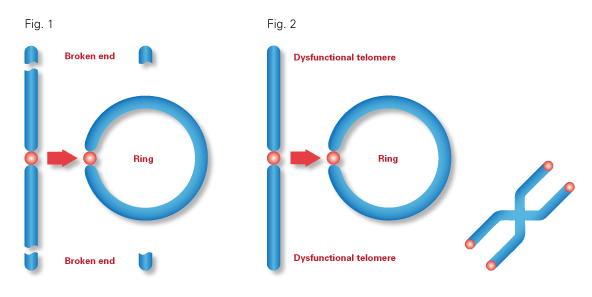
A ring chromosome is a chromosome in which both ends have been deleted and the two broken ends have reunited to form a ring-shape. Ring chromosomes may form in cells following genetic damage by mutagens like radiation, but they may also arise spontaneously during development.
Genetic information is often moved or deleted when a ring chromosome forms, as a result
genes on that chromosome may not be correctly expressed. This can lead to health problems which range from congenital conditions to cancer.
Ring chromosomes are very rare, but have been found in nearly all human chromosomes. Some people have ring chromosomes in their bodies and remain unaffected. Others may be identified shortly after birth. The spectrum swings from profound developmental delays to a blissfully unaware existence, illustrating the diversity of human genetics.
|
 |
|
|
|
Stem cells may correct abnormal chromosomes
Pluripotent stem cell therapy may some day correct abnormal chromosome defects of ring chromosomes.
Geneticists from Ohio, California and Japan joined forces in a quest to correct a faulty chromosome through cell reprogramming. Their study, published online today in Nature, used stem cells to correct a defective "ring chromosome" with a normal chromosome. Such therapy has the promise to correct chromosome abnormalities that give rise to birth defects, mental disabilities and growth limitations.
"In the future, it may be possible to use this approach to take cells from a patient that has a defective chromosome with multiple missing or duplicated genes and rescue those cells by removing the defective chromosome and replacing it with a normal chromosome."
Anthony Wynshaw-Boris, MD, PhD, James H. Jewell MD '34 Professor of Genetics and chair of Case Western Reserve School of Medicine Department of Genetics and Genome Sciences and University Hospitals Case Medical Center, and senior author.
Wynshaw-Boris led this research while a professor in pediatrics, the Institute for Human Genetics and the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research at UC, San Francisco (UCSF) before joining the faculty at Case Western Reserve in June 2013.
Individuals with ring chromosomes may display a variety of birth defects, but nearly all persons with ring chromosomes at least display short stature due to problems with cell division.
A normal chromosome is linear, with its ends protected, but with ring chromosomes, the two ends of the chromosome fuse together, forming a circle.
This fusion can be associated with large terminal deletions, a process where portions of the chromosome or DNA sequences are missing. These deletions can result in disabling genetic disorders if the genes in the deletion are necessary for normal cellular functions.
The prospect for effective counter measures has evaded scientists—until now.
The international research team discovered the potential for substituting the malfunctioning ring chromosome with an appropriately functioning one during reprogramming of patient cells into induced pluripotent stem cells (iPSCs). iPSC reprogramming is a technique that was developed by Shinya Yamanaka, MD, PhD, a co-corresponding author on the Nature paper. Yamanaka is a senior investigator at the UCSF-affiliated Gladstone Institutes, a professor of anatomy at UCSF, and the director of the Center for iPS Cell Research and Application (CiRA) at the Institute for Integrated Cell-Material Sciences (iCeMS) in Kyoto University. He won the Nobel Prize in Medicine in 2012 for developing the reprogramming technique.
Marina Bershteyn, PhD, a postdoctoral fellow in the Wynshaw-Boris lab at UCSF, along with Yohei Hayashi, PhD, a postdoctoral fellow in the Yamanaka lab at the Gladstone Institutes, reprogrammed skin cells from three patients with abnormal brain development due to a rare disorder called Miller Dieker Syndrome.
Miller Dieker Syndrome results from large terminal deletions in one arm of chromosome 17. One patient had a ring chromosome 17 with the deletion and two other patients had large terminal deletions in one of their chromosome 17 — but not a ring. Additionally, each patient had one normal chromosome 17.
Researchers observed that after reprogramming, the ring chromosome 17 with the deletion vanished entirely — replaced by a duplicated copy of the normal chromosome 17. However, the terminal deletions in the other two patients remained after reprogramming. To make sure this phenomenon was not unique to ring chromosome 17, researchers reprogrammed cells from two different patients each having ring chromosomes 13. These reprogrammed cells also lost the ring chromosome, and contained a duplicated copy of the normal chromosome 13.
"It appears that ring chromosomes are lost during rapid and continuous cell divisions during reprogramming. The duplication of the normal chromosome then corrects for that lost chromosome."
Shinya Yamanaka, MD, PhD, senior investigator, UCSF-affiliated Gladstone Institutes, professor of anatomy, UCSF, director Center for iPS Cell Research and Application (CiRA), Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Nobel Prize in Medicine, 2012 for developing reprogramming technique, co-corresponding author on the Nature paper.
"Ring loss and duplication of whole chromosomes occur with a certain frequency in stem cells," explained Bershteyn. "When chromosome duplication compensates for the loss of the corresponding ring chromosome with a deletion, this provides a possible avenue to correct large-scale problems in a chromosome that have no chance of being corrected by any other means."
"It is likely that our findings apply to other ring chromosomes, since the loss of the ring chromosome occurred in cells reprogrammed from three different patients," said Hayashi.
"In theory, the way you could potentially correct a chromosome with deletions or duplications is to make a ring out of it and then get rid of the ring chromosome during reprogramming.
"Ring chromosomes are quite rare, but chromosome abnormalities are much more common and cause a variety of severe birth defects.
"So far, it is only possible to do this chromosome therapy for cells in culture, not in human beings. However, it may be useful to use this for tissue repair of birth defects and other abnormalities found in individuals with chromosomal abnormalities as techniques for regenerative medicine are developed in the future."
Anthony Wynshaw-Boris, MD, PhD
Abstract
Ring chromosomes are structural aberrations commonly associated with birth defects, mental disabilities and growth retardation1, 2. Rings form after fusion of the long and short arms of a chromosome, and are sometimes associated with large terminal deletions2. Owing to the severity of these large aberrations that can affect multiple contiguous genes, no possible therapeutic strategies for ring chromosome disorders have been proposed. During cell division, ring chromosomes can exhibit unstable behaviour leading to continuous production of aneuploid progeny with low viability and high cellular death rate3, 4, 5, 6, 7, 8, 9. The overall consequences of this chromosomal instability have been largely unexplored in experimental model systems. Here we generated human induced pluripotent stem cells (iPSCs)10, 11, 12 from patient fibroblasts containing ring chromosomes with large deletions and found that reprogrammed cells lost the abnormal chromosome and duplicated the wild-type homologue through the compensatory uniparental disomy (UPD) mechanism. The karyotypically normal iPSCs with isodisomy for the corrected chromosome outgrew co-existing aneuploid populations, enabling rapid and efficient isolation of patient-derived iPSCs devoid of the original chromosomal aberration. Our results suggest a fundamentally different function for cellular reprogramming as a means of ‘chromosome therapy’13 to reverse combined loss-of-function across many genes in cells with large-scale aberrations involving ring structures. In addition, our work provides an experimentally tractable human cellular system for studying mechanisms of chromosomal number control, which is of critical relevance to human development and disease.
These authors contributed equally to this work:
Marina Bershteyn & Yohei Hayashi
Other collaborators on the paper included Guillaume Desachy, M.Sc., Edward C. Hsiao, MD, Salma Sami, Kathryn M. J. Tsang, and Lauren A. Weiss, PhD, of UCSF; and Arnold R. Kriegstein, MD, PhD of the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research at UCSF.
About Case Western Reserve University School of Medicine
Founded in 1843, Case Western Reserve University School of Medicine is the largest medical research institution in Ohio and is among the nation's top medical schools for research funding from the National Institutes of Health. The School of Medicine is recognized throughout the international medical community for outstanding achievements in teaching. The School's innovative and pioneering Western Reserve2 curriculum interweaves four themes--research and scholarship, clinical mastery, leadership, and civic professionalism--to prepare students for the practice of evidence-based medicine in the rapidly changing health care environment of the 21st century. Nine Nobel Laureates have been affiliated with the School of Medicine.
Annually, the School of Medicine trains more than 800 MD and MD/PhD students and ranks in the top 25 among U.S. research-oriented medical schools as designated by U.S. News & World Report's "Guide to Graduate Education."
The School of Medicine's primary affiliate is University Hospitals Case Medical Center and is additionally affiliated with MetroHealth Medical Center, the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, and the Cleveland Clinic, with which it established the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University in 2002. http://casemed.case.edu
About UCSF
UCSF is a leading university dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. It includes top-ranked graduate schools of dentistry, medicine, nursing and pharmacy, a graduate division with nationally renowned programs in basic biomedical, translational and population sciences, as well as a preeminent biomedical research enterprise and two top-ranked hospitals, UCSF Medical Center and UCSF Benioff Children's Hospital.
|
|
