|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive April 3, 2014
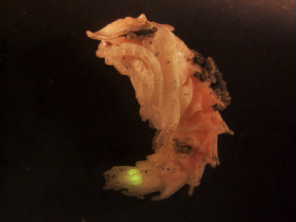
A firefly's photic organ is functional throughout the pupa stage but
glows only
when the pupa is disturbed. | Photo by Armin Moczek and Matthew Stansbury
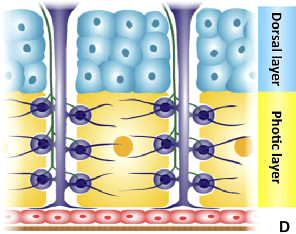
A cross-section diagram of the adult firefly lantern shows photic (yellow) and dorsal (blue) layers, tracheae and tracheal end-cell complexes (purple), nerve axons (green), epidermis (red), the transparent cuticle (brown), and the clustered cells of the photic layer (orange circle). | Photo by Matthew Stansbury, based on R.F. Chapman after D.S. Smith 1963 |
Researchers identified two parallel mechanisms for retaining memories:
the protein adducin stimulates the growth of synapses - helping retain memory;
but, the musashi protein inhibits the stabilization of synapses facilitating memory loss.
Balance between these two proteins is crucial for keeping memories.
Image Credit: Cell, Volume 156, Issue 6, 1153-1166, 13 March 2014.
|
 |
|
|
|
Hox genes determine body form and firefly light
Nothing had been known about the genetics or evolution of the firefly's lantern, until now. For the first time — its luminescent organ and the ancient genes used in its formation, are being understood. And Hox genes are a large part of the story.
Hox genes are famous for defining body regions and their boundaries — such as the cervical, thoracic and lumbar vertebrae of mammals, or the head, thorax and abdomen of insects.
Hox genes are also famous for specifying which appendage will form on a given insect segment, such as a mouthpart on the head and a wing on the thorax. The firefly, a beetle, has a forewing that became a hardened shell rather than a membrane, due to the action of a Hox gene. What is nearly unprecedented, though, is that this beetle belongs to a family of about 2,000 species, yet its two Hox genes alone — abdominal-A (abd-A) and Abdominal-B (Abd-B) — have the ability to regulate an entirely new and complex organ found in no other beetle: its luminescent lantern.
“Our study demonstrates unprecedented roles for two Hox genes.
"Hox genes have been highly preserved over evolutionary times to lay out the basic body forms of many animals, from humans to flies. On top of that, Hox genes have recently acquired control over important aspects of lantern development.
“The notion that Hox genes can simply acquire new responsibilities quickly, especially without compromising old ones, is a new idea.
“The lantern is one of relatively few examples showing that this gene flexibility occurs in animal evolution.”
Armin Moczek, associate professor in the College of Arts and Sciences, Department of Biology, paper co-author along with his former Ph.D. student Matthew Stansbury, now at the University of Arizona.
What Stansbury and Moczek found by turning off the function of each of these two genes was that both are required for proper photic organ formation.
“Particularly, the Abd-B Hox gene is critical to all aspects of photic organ formation, suggesting that it evolved novel lantern-specific regulatory functions,” Moczek said.
Stansbury and Moczek also found that Abd-B repressed pigmentation in the abdomin of fireflies, creating the translucent cuticle which allows light to escape from the body's interior.
The sixth and seventh abdominal segments of most insects ordinarily are controlled by the abd-A Hox gene— and are precisely the abdominal segments housing the fireflies lantern. This suggests that at some point there was an anterior expansion of Abd-B control functions. This is a big deal because Hox genes, which are very important to establishing basic body plans, normally have strict boundaries of gene expression [the "turning on" of genes].
“This is just the beginning. We want to know the target genes that interact with abd-A and Abd-B to form organs. We want to know exactly when and where a given gene product becomes active. And, we want to learn more about how the adult organ evolves from the larval organ, a much simpler structure located on a different body segment than the adult form.”
Armin Moczek, PhD
Hox-gene innovation — truely, unique innovation — is more widespread than generally thought. Another example can be found in beetle horns, one of Moczek’s primary areas of research. The horns of beetles do not correspondence to traits in other beetles or insects — and thus are novel.
Work conducted by former graduate student Bethany Wasik found that beetle horns grow out of the thorax of beetles and are regulated in part by the Hox gene known as Sex-combs-reduced (Scr). Scr has acquired new functions over its long history, including pre-pupal growth and pupal remodeling. This suggests that Scr can diversify quickly over remarkedly short evolutionary distance. So while maintaining morphologic pattern definitions, the Hox complex of genes also plays and intergral role in developing novel and complex new traits.
Novel traits are well-understood by Moczek, and Hox genes are especially close to the Indiana University (IU) Department of Biology. The homeobox (Hox is the abbreviation) DNA sequence was discovered in 1983 by students in the laboratory of IU Distinguished Professor of Biology —Thom Kaufman.
The paper explaining this discovery is titled “The function of Hox and appendage-patterning genes in the development of an evolutionary novelty, the Photuris firefly lantern,” written by Matthew Stansbury, University of Arizona, and Armin Moczek, Indiana University, and is published in the Proceedings of the Royal Society B.
Abstract
Uncovering the mechanisms underlying the evolution of novel traits is a central challenge in biology. The lanterns of fireflies are complex traits that lack even remote homology to structures outside luminescent beetle families. Representing unambiguous novelties by the strictest definition, their developmental underpinnings may provide clues to their origin and offer insights into the mechanisms of innovation in developmental evolution. Lanterns develop within the context of abdominal Hox expression domains, and we hypothesized that lantern formation may be instructed in part by these highly conserved transcription factors. We show that transcript depletion of Abdominal-B inPhoturis fireflies results in extensive disruption of the adult lantern, suggesting that the evolution of adult lanterns involved the acquisition of a novel regulatory role for this Hox gene. Using the same approach, we show that the Hox geneabdominal-A may control important secondary aspects of lantern development. Lastly, we hypothesized that lantern evolution may have involved the recruitment of dormant abdominal appendage-patterning domains; however, transcript depletion of two genes, Distal-less and dachshund, suggests that they do not contribute to lantern development. Our results suggest that complex novelties can arise within the confines of ancestral regulatory landscapes through acquisition of novel targets without compromising ancestral functions.
|
|
