|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive July 16, 2014
Nitric oxide (NO) modification — a process known as S-nitrosylation — regulates
important functions throughout the body under normal conditions.
But with aging, environmental toxins, or stress-related injuries, abnormal
S-nitrosylation reactions can occur and contribute to disease.
|
|
|
An amazing'switch' in our brains
An amazing "switch" may be available to shut off (and turn on?) signals that promote the generation of new nerve cells and survival of old ones.
A new study by researchers at Sanford-Burnham Medical Research Institute (Sanford-Burnham) has identified a chemical "switch" that controls both the generation of new neurons from neural stem cells and the survival of existing nerve cells in the brain.
The work was published July 3 in Cell Reports.
The new work suggests that the MEF2 gene is a chemical switch that may be a potential therapeutic target to protect against neurons being lost to a variety of diseases, such as Alzheimer's, Parkinson's and autism.
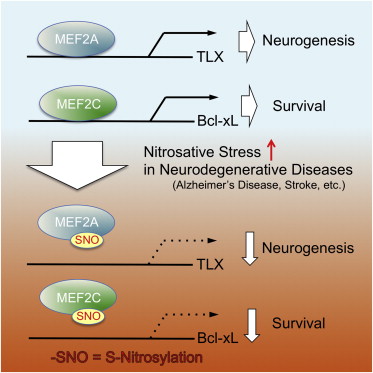
"We have shown that when nitric oxide (NO)—a highly reactive free radical [an atom or molecule that is unpaired to another]—reacts with MEF2 it makes it so that MEF2 can no longer bind to and activate the genes that drive neurogenesis and neural survival.
"What's unique here is that a single alteration to MEF2 controls two distinct events—the generation of new neurons and the survival of existing neurons."
Stuart Lipton, MD, PhD, practicing clinical neurologist, senior author, director and professor, Neuroscience and Aging Research Center, Sanford-Burnham Medical Research Institute.
In the brain, transcription factors are critical for linking external stimuli to the production of proteins, a process which enables neurons to adapt to changing environments. Members of the MEF2 family of transcription factors plays an important role in neurogenesis and neural survival, as well as in the processes of learning and memory. Mutations in the MEF2 gene have been associated with a range of neurodegenerative disorders, including Alzheimer's and autism.
The process of NO-protein modifications — also known as S-nitrosylation — was first described by Lipton and collaborators 20 years ago.
S-nitrosylation has important regulatory functions under normal physiologic conditions throughout the body. But with aging, environmental toxins, or stress-related injuries, abnormal S-nitrosylation reactions can occur and contribute to disease.
"Our laboratory had previously shown that S-nitrosylation of the MEF2 gene, controlled neural survival in Parkinson's disease. Now we have shown that this same reaction is more wide spread, occurring in other neurologic conditions such as stroke, autism and Alzheimer's disease.
"While the major gene targets of MEF2 may be different in various diseases and brain areas, the remarkable new finding here is that we may be able to treat each of these neurologic disorders by preventing a common S-nitrosylation modification in MEF2.
"The findings suggest that the development of a small therapeutic molecule — one that can cross the blood-brain barrier and block S-nitrosylation of MEF2, or in some other way increase MEF2 transcriptional activity — could promote new brain cell growth and protect existing cells in several neurodegenerative disorders.
"We have already found several such molecules in our high-throughput screening and drug discovery efforts, so the potential for developing new drugs to attack this pathway is very exciting."
Stuart Lipton, MD, PhD
Highlights
•S-Nitrosylation of MEF2 at conserved cysteine residues occurs in plants and animal
•S-Nitrosylation of MEF2 inhibits its DNA binding and transcriptional activity
•S-Nitrosylation of MEF2 inhibits both neurogenesis and neuronal survival
Summary
Redox-mediated posttranslational modifications represent a molecular switch that controls major mechanisms of cell function. Nitric oxide (NO) can mediate redox reactions via S-nitrosylation, representing transfer of an NO group to a critical protein thiol. NO is known to modulate neurogenesis and neuronal survival in various brain regions in disparate neurodegenerative conditions. However, a unifying molecular mechanism linking these phenomena remains unknown. Here, we report that S-nitrosylation of myocyte enhancer factor 2 (MEF2) transcription factors acts as a redox switch to inhibit both neurogenesis and neuronal survival. Structure-based analysis reveals that MEF2 dimerization creates a pocket, facilitating S-nitrosylation at an evolutionally conserved cysteine residue in the DNA binding domain. S-Nitrosylation disrupts MEF2-DNA binding and transcriptional activity, leading to impaired neurogenesis and survival in vitro and in vivo. Our data define a molecular switch whereby redox-mediated posttranslational modification controls both neurogenesis and neurodegeneration via a single transcriptional signaling cascade.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
About Sanford-Burnham Medical Research Institute
Sanford-Burnham Medical Research Institute is dedicated to discovering the fundamental molecular causes of disease and devising the innovative therapies of tomorrow. Sanford-Burnham takes a collaborative approach to medical research with major programs in cancer, neurodegeneration and stem cells, diabetes, and infectious, inflammatory, and childhood diseases. The Institute is recognized for its National Cancer Institute-designated Cancer Center, its NIH-designated Neuroscience Center Cores, and expertise in drug discovery technologies. Sanford-Burnham is a nonprofit, independent institute that employs more than 1,000 scientists and staff in San Diego (La Jolla), Calif., and Orlando (Lake Nona), Fla. For more information, visit us at sanfordburnham.org.
Sanford-Burnham can also be found on Facebook at facebook.com/sanfordburnham and on Twitter @sanfordburnham.
Return to top of page |
