|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive Nov 26, 2014
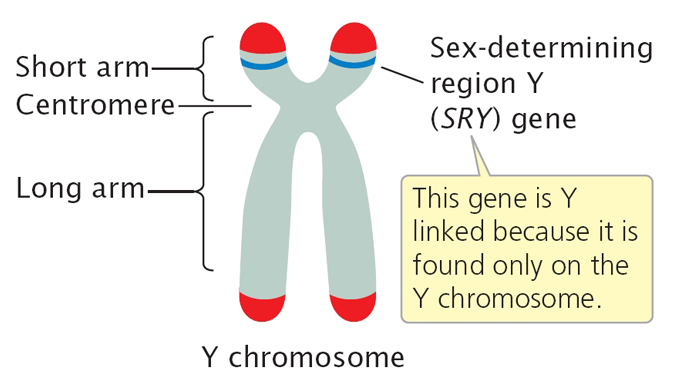
Sexual differentiation begins around the seventh week of gestation, with the SRY gene on the Y chromosome initiating the process for males. |
|
|
|
|
|
A gene mutation that alters the male fetus
Case Western Reserve researchers have identified a mutation in SRY protein that alters male gonad formation before birth.
This discovery marks the latest in a series of new findings on how the SRY (sex-determining region on the Y chromosome) protein works. SRY is a master switch for typical male gonad formation. However, a newly identified SRY mutation prevents the protein from folding exactly as it should — resulting in impaired gonad formation.
The research results appear in the November 21 edition of the Journal of Biological Chemistry, where it was named Paper of the Week. Joseph Racca is a Case Western Reserve biochemistry doctoral student and senior author on the paper. In his description of SRY’s infrastructure, he describes SRY in terms typically reserved for the architecture of historic European churches.
“The mutation occurs in a strategic part of the SRY protein that we describe as a ‘buttress’ — molecular struts and girders that function to support an angular DNA-bending surface. Just as the flying buttresses of Gothic cathedrals ensure structural stability, the SRY buttress is responsible for proper protein folds and stability.”
Joseph Racca, student Case Western Reserve, senior author.
Racca is a student in the laboratory of Michael Weiss, MD, PhD, chair of the department of biochemistry and paper co-author. Weiss has led a team of scientists through multiple discoveries of unique SRY protein mutations, each having its own affect on SRY structure and function.
“The DNA-bending module of the SRY protein is preserved in a large family of proteins which direct development, including how stem cells form the brain and other organs,” adds Michael Weiss PhD, distinguished research professor, chair of biochemistry and a co-author of this paper.
Mutations in the SRY protein take place
within an L-shaped protein known as an
HMG box — or 'High Mobility Group."
The HMG box is a structure containing
proteins that regulate DNA transcription,
replication, recombination and repair.
Mutations in the HMG box place a hole in the hinge of the L shaped protein, resulting in the structure's collapse. A collapse leads to incomplete protein folding and binding of the DNA which affects all the various functions of male sex determination in humans.
Joseph Racca:“At the start of this project, we speculated that this SRY mutation would be unfavorable. But we underestimated the extent and range of impairments. This ‘perfect storm’ leads to the collapse of almost all the molecular functions of SRY, including cell movement and stability, DNA recognition and gene regulation.”
Mutations in SRY alter male development in fetal life. In some cases, these mutations can also lead to XY sex reversal, a syndrome in which male babies are born with a female appearance, including external genitalia, a uterus and fallopian tubes. But when these patients fail to menstruate at the onset of puberty, an XY chromosome is discovered. This condition is known as Swyer’s syndrome (46, XY pure gonadal dysgenesis with female somatic phenotype) and affects 1 in 80,000 births.
SRY mutations cause a number of disorders including pseudohermaphroditism — 1 in 99,000 births — with partial ovarian and testicular differentiation. Impaired differentiation in gonads increases a child's risk for Gonadoblastoma, a pediatric cancer.
In the next round of research, investigators want to link the SRY database to other SOX (Sry-related HMG Box) transcription factors and further evaluate SRY protein function in stem-cell biology and cancer.
Capsule
Background: Testis-determining factor SRY provides a model of bent protein·DNA complex.
Results: Mutation of an invariant Trp perturbs multiple biochemical, cellular, and transcriptional activities.
Conclusion: Folding and function of a sequence-specific HMG box require a core “aromatic buttress.”
Significance: Mutations in SRY causing human sex reversal probe the architecture and evolution of a DNA-bending motif.
Abstract
Human testis determination is initiated by SRY, a Y-encoded architectural transcription factor. Mutations in SRY cause 46 XY gonadal dysgenesis with female somatic phenotype (Swyer syndrome) and confer a high risk of malignancy (gonadoblastoma). Such mutations cluster in the SRY high mobility group (HMG) box, a conserved motif of specific DNA binding and bending. To explore structure-function relationships, we constructed all possible substitutions at a site of clinical mutation (W70L). Our studies thus focused on a core aromatic residue (position 15 of the consensus HMG box) that is invariant among SRY-related HMG box transcription factors (the SOX family) and conserved as aromatic (Phe or Tyr) among other sequence-specific boxes. In a yeast one-hybrid system sensitive to specific SRY-DNA binding, the variant domains exhibited reduced (Phe and Tyr) or absent activity (the remaining 17 substitutions). Representative nonpolar variants with partial or absent activity (Tyr, Phe, Leu, and Ala in order of decreasing side-chain volume) were chosen for study in vitro and in mammalian cell culture. The clinical mutation (Leu) was found to markedly impair multiple biochemical and cellular activities as respectively probed through the following: (i) in vitro assays of specific DNA binding and protein stability, and (ii) cell culture-based assays of proteosomal degradation, nuclear import, enhancer DNA occupancy, and SRY-dependent transcriptional activation. Surprisingly, however, DNA bending is robust to this or the related Ala substitution that profoundly impairs box stability. Together, our findings demonstrate that the folding, trafficking, and gene-regulatory function of SRY requires an invariant aromatic “buttress” beneath its specific DNA-bending surface.
Collaborating with Weiss and Racca on this SRY mutation research were Yen-Shan Chen, PhD, James D. Maloy, Nalinda Wickramasinghe, PhD, and associate professor Nelson B. Phillips, PhD, all of the Department of Biochemistry, Case Western Reserve University School of Medicine.
This work was supported in part by a grant to Michael A. Weiss, MD, from the National Institutes of Health (GM080505) and represents a contribution from the Cleveland Center for Membrane & Structural Biology. Patricia K. Donahoe, MD, of the Massachusetts General Hospital and Harvard Medical School, Boston, generously provided rodent embryonic CH34 cells for the project.
About Case Western Reserve University School of Medicine
Founded in 1843, Case Western Reserve University School of Medicine is the largest medical research institution in Ohio and is among the nation’s top medical schools for research funding from the National Institutes of Health. The School of Medicine is recognized throughout the international medical community for outstanding achievements in teaching. The School’s innovative and pioneering Western Reserve2 curriculum interweaves four themes--research and scholarship, clinical mastery, leadership, and civic professionalism--to prepare students for the practice of evidence-based medicine in the rapidly changing health care environment of the 21st century. Nine Nobel Laureates have been affiliated with the School of Medicine.
Annually, the School of Medicine trains more than 800 MD and MD/PhD students and ranks in the top 25 among U.S. research-oriented medical schools as designated by U.S. News & World Report’s “Guide to Graduate Education.”
The School of Medicine’s primary affiliate is University Hospitals Case Medical Center and is additionally affiliated with MetroHealth Medical Center, the Louis Stokes Cleveland Department of Veterans Affairs Medical Center, and the Cleveland Clinic, with which it established the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University in 2002. http://casemed.case.edu
Return to top of page |
