|
|
Welcome to The Visible Embryo, a comprehensive educational resource on human development from conception to birth.
The Visible Embryo provides visual references for changes in fetal development throughout pregnancy and can be navigated via fetal development or maternal changes.
The National Institutes of Child Health and Human Development awarded Phase I and Phase II Small Business Innovative Research Grants to develop The Visible Embryo. Initally designed to evaluate the internet as a teaching tool for first year medical students, The Visible Embryo is linked to over 600 educational institutions and is viewed by more than one million visitors each month.
Today, The Visible Embryo is linked to over 600 educational institutions and is viewed by more than 1 million visitors each month. The field of early embryology has grown to include the identification of the stem cell as not only critical to organogenesis in the embryo, but equally critical to organ function and repair in the adult human. The identification and understanding of genetic malfunction, inflammatory responses, and the progression in chronic disease, begins with a grounding in primary cellular and systemic functions manifested in the study of the early embryo.

The World Health Organization (WHO) has created a new Web site to help researchers, doctors and patients obtain reliable information on high-quality clinical trials. Now you can go to one website and search all registers to identify clinical trial research underway around the world!

|
|
| Disclaimer: The Visible Embryo web site is provided for your general information only. The information contained on this site should not be treated as a substitute for medical, legal or other professional advice. Neither is The Visible Embryo responsible or liable for the contents of any websites of third parties which are listed on this site. |
|
|
 |
|
Content protected under a Creative Commons License. Commons License.
No dirivative works may be made or used for commercial purposes. |
|
|
| |
|
|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
|
|
|
|
Home | Pregnancy Timeline | News Alerts |News Archive May 11, 2015
Like two sides of a highway, arteries and veins are kept adjacent and
parallel to one another in order to regulate our body temperature.
Image Credit: Developmental Cell |
|
|
|
|
|
How come we are warm-blooded?
Our arteries and veins run parallel to one another in order to regulate our body temperature.
Any student of mammalian anatomy and physiology knows that arteries carry blood away from the heart and veins carry blood toward the heart. Like two sides of a highway, these blood vessels are relatively adjacent and parallel to one another.
Blood delivery begins with the formation of blood vessels from the mesoderm layer of the early three layered embryo. The process is driven by the recruitment of undifferentiated mesodermal cells into the endothelial layer of cells, where they line the blood vessels. The endothelial cells go on to migrate into almost every region of the body. The formation of new blood vessels occurs from the endothelial cells within the blood vessel walls. |
|
Without this parallel system of mammalian arteries and veins, we would probably not be able to regulate our own body temperature. This is a key feature of being warm-blooded in mammals. In Developmental Cell, a collaboration of Japanese and German researchers report that, in both cold and hot conditions, mice without aligned veins and arteries are unable to regulate their body temperature efficiently.
The authors investigated the molecular mechanisms that control this alignment believing that our vein to artery configuration might be a recent adaptation. Their belief is based on the observation that "lower" animals — such as fish and reptiles — which precede mammals in evolutionary history, lack parallel veins and arteries and are thus cold-blooded. Without parallel blood vessel alignment, there can be no regulation of body temperature through heat exchanged between the two vessels.
The dual system constitutes a means of thermoregulation of our body temperature. Blood vessel alignment is an elegant interplay to reduce body heat loss.
Highlights
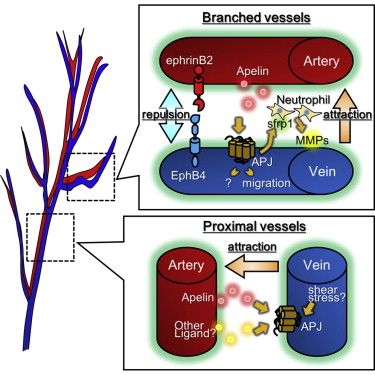
• Apelin or APJ mutant mice have abnormal arterial-venous (A-V) alignment
• Apelin from arterial endothelial cells (ECs) induces chemotaxis of APJ+ venous ECs
• Venous ECs induce neutrophil production of MMP-9, allowing for EC migration
• Mice with defective A-V alignment have defects in thermoregulation
Abstract
Molecular pathways regulating the development of arterial and venous endothelial cells (ECs) are now well established, but control of parallel arterial-venous alignment is unclear. Here we report that arterial-venous alignment in the skin is determined by apelin receptor (APJ) expression in venous ECs. One of the activators of APJ is apelin. We found that apelin is produced by arterial ECs during embryogenesis, induces chemotaxis of venous ECs, and promotes the production of secreted Frizzled-related protein 1 (sFRP1) by APJ+ ECs. sFRP1 stimulates matrix metalloproteinase production by Ly6B.2+ neutrophil-like cells located between the arteries and veins, resulting in remodeling of extracellular matrices to support venous displacement. Moreover, using apelin- or APJ-deficient mice, which exhibit arterial-venous disorganization, we found that arterial-venous alignment is involved in thermoregulation, possibly by regulating countercurrent heat exchange. We hypothesize that the evolution of parallel juxtapositional arterial-venous alignment was an adaptation to reduce body heat loss.
Kidoya et al.: "APJ Regulates Parallel Alignment of Arteries and Veins in the Skin" Developmental Cell. (April 23, 2015) http://dx.doi.org/10.1016/j.devcel.2015.02.024
Return to top of page
|
